Difference in Immunogenic Responses to COVID-19 Vaccines in Patients With Cancer Receiving Chemotherapy Versus Nonchemotherapy Treatment
- PMID: 36821802
- PMCID: PMC10166411
- DOI: 10.1200/GO.22.00331
Difference in Immunogenic Responses to COVID-19 Vaccines in Patients With Cancer Receiving Chemotherapy Versus Nonchemotherapy Treatment
Abstract
Purpose: The COVID-19 pandemic has affected public health worldwide. The efficacy and safety of COVID-19 vaccines have been evaluated in the general population; however, data on patients with malignancies are limited.
Methods: This prospective longitudinal observational cohort study was conducted between June and July 2021. Enrolled adult patients with cancer were divided into chemotherapy and nonchemotherapy groups. All participants were immunized with two doses of the ChAdOx1 nCoV-19 or CoronaVac COVID-19 vaccines. The primary outcome was a comparison of the immunogenicity (as assessed by spike protein [anti-S] immunoglobulin G [IgG] antibody titers) of two doses of COVID-19 vaccine in the chemotherapy and nonchemotherapy groups. The secondary outcomes included the anti-S IgG seroconversion rate and vaccine safety in both groups.
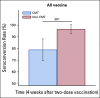
Results: Among the 173 enrolled patients with solid cancer, after COVID-19 vaccination, the chemotherapy group had a significantly lower median anti-S IgG titer than the nonchemotherapy group (26 v 237 U/mL, P < .001). A statistically significant difference in anti-S IgG titer was found between groups vaccinated with CoronaVac (7 v 90 U/mL, P < .001), but no difference was found in those vaccinated with ChAdOx1 nCoV-19 (818 v 1061 U/mL, P = .075). The anti-S IgG seroconversion rate was significantly lower in the chemotherapy group than that in the nonchemotherapy group (78.9% v 96.5%, P = .001). No new or serious vaccine-related adverse events were reported.
Conclusion: Patients with solid cancer receiving a COVID-19 vaccine while undergoing chemotherapy had lower immunogenicity responses to vaccination than those who were vaccinated while undergoing nonchemotherapy treatment. No statistically significant difference was observed in the COVID-19 vaccine safety profiles between groups.
Trial registration: ClinicalTrials.gov TCTR20221001004.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures



References
-
- World Health Organization : Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. https://www.who.int/publications/m/item/weekly-epidemiological-update-on...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

