Functional Engagement of the PD-1/PD-L1 Complex But Not PD-L1 Expression Is Highly Predictive of Patient Response to Immunotherapy in Non-Small-Cell Lung Cancer
- PMID: 36821809
- PMCID: PMC10414696
- DOI: 10.1200/JCO.22.01748
Functional Engagement of the PD-1/PD-L1 Complex But Not PD-L1 Expression Is Highly Predictive of Patient Response to Immunotherapy in Non-Small-Cell Lung Cancer
Abstract
Purpose: In many cancers, the expression of immunomodulatory ligands leads to immunoevasion, as exemplified by the interaction of PD-L1 with PD-1 on tumor-infiltrating lymphocytes. Profound advances in cancer treatments have come with the advent of immunotherapies directed at blocking these immuno-suppressive ligand-receptor interactions. However, although there has been success in the use of these immune checkpoint interventions, correct patient stratification for these therapies has been challenging.
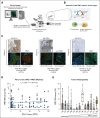
Materials and methods: To address this issue of patient stratification, we have quantified the intercellular PD-1/PD-L1 interaction in formalin-fixed paraffin-embedded tumor samples from patients with non-small cell lung carcinoma, using a high-throughput automated quantitative imaging platform (quantitative functional proteomics [QF-Pro]).
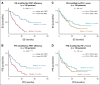
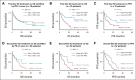
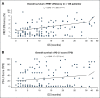
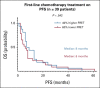
Results: The multisite blinded analysis across a cohort of 188 immune checkpoint inhibitor-treated patients demonstrated the intra- and intertumoral heterogeneity of PD-1/PD-L1 immune checkpoint engagement and notably showed no correlation between the extent of PD-1/PD-L1 interaction and PD-L1 expression. Importantly, PD-L1 expression scores used clinically to stratify patients correlated poorly with overall survival; by contrast, patients showing a high PD-1/PD-L1 interaction had significantly better responses to anti-PD-1/PD-L1 treatments, as evidenced by increased overall survival. This relationship was particularly strong in the setting of first-line treatments.
Conclusion: The functional readout of PD-1/PD-L1 interaction as a predictive biomarker for the stratification of patients with non-small-cell lung carcinoma, combined with PD-L1 expression, should significantly improve the response rates to immunotherapy. This would both capture patients excluded from checkpoint immunotherapy (high PD-1/PD-L1 interaction but low PD-L1 expression, 24% of patients) and additionally avoid treating patients who despite their high PD-L1 expression do not respond and suffer from side effects.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Savic Prince S, Bubendorf L: Predictive potential and need for standardization of PD-L1 immunohistochemistry. Virchows Arch 474:475-484, 2019 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

