DNA-initiated epigenetic cascades driven by C9orf72 hexanucleotide repeat
- PMID: 36822200
- PMCID: PMC10121948
- DOI: 10.1016/j.neuron.2023.01.022
DNA-initiated epigenetic cascades driven by C9orf72 hexanucleotide repeat
Erratum in
-
DNA-initiated epigenetic cascades driven by C9orf72 hexanucleotide repeat.Neuron. 2023 Apr 19;111(8):1345. doi: 10.1016/j.neuron.2023.03.035. Neuron. 2023. PMID: 37080168 Free PMC article. No abstract available.
Abstract
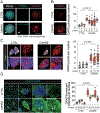
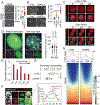
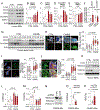
The C9orf72 hexanucleotide repeat expansion (HRE) is the most frequent genetic cause of the neurodegenerative diseases amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Here, we describe the pathogenic cascades that are initiated by the C9orf72 HRE DNA. The HRE DNA binds to its protein partner DAXX and promotes its liquid-liquid phase separation, which is capable of reorganizing genomic structures. An HRE-dependent nuclear accumulation of DAXX drives chromatin remodeling and epigenetic changes such as histone hypermethylation and hypoacetylation in patient cells. While regulating global gene expression, DAXX plays a key role in the suppression of basal and stress-inducible expression of C9orf72 via chromatin remodeling and epigenetic modifications of the promoter of the major C9orf72 transcript. Downregulation of DAXX or rebalancing the epigenetic modifications mitigates the stress-induced sensitivity of C9orf72-patient-derived motor neurons. These studies reveal a C9orf72 HRE DNA-dependent regulatory mechanism for both local and genomic architectural changes in the relevant diseases.
Keywords: ALS; C9orf72; DAXX; DNA; FTD; chromatin; epigenetic; neurodegeneration; phase separation; transcription.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
The DAXX tax: C9orf72 DNA repeat expansions drive gain- and loss-of-function pathology in c9FTD/ALS.Neuron. 2023 Apr 19;111(8):1165-1167. doi: 10.1016/j.neuron.2023.03.028. Neuron. 2023. PMID: 37080165
References
-
- DeJesus-Hernandez M, Mackenzie Ian R., Boeve Bradley F., Boxer Adam L., Baker M, Rutherford Nicola J., Nicholson Alexandra M., Finch NiCole A., Flynn H, Adamson J, et al. (2011). Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 72, 245–256. 10.1016/j.neuron.2011.09.011. - DOI - PMC - PubMed
-
- Renton Alan E., Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick Jennifer C., Laaksovirta H, van Swieten John C., Myllykangas L, et al. (2011). A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron 72, 257–268. 10.1016/j.neuron.2011.09.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

