Naltrexone plus bupropion reduces cigarette smoking in individuals with methamphetamine use disorder: A secondary analysis from the CTN ADAPT-2 trial
- PMID: 36822269
- PMCID: PMC11008704
- DOI: 10.1016/j.josat.2023.208987
Naltrexone plus bupropion reduces cigarette smoking in individuals with methamphetamine use disorder: A secondary analysis from the CTN ADAPT-2 trial
Abstract
Introduction: Methamphetamine (MA) use is marked by high rates of comorbid tobacco smoking, which is associated with more severe drug use and worse clinical outcomes compared to single use of either drug. Research has shown the combination of naltrexone plus oral bupropion (NTX-BUP) improves smoking cessation outcomes in non-MA-using populations. In the Accelerated Development of Additive Pharmacotherapy Treatment (ADAPT-2) study, NTX-BUP successfully reduced MA use. Our aim in this secondary data analysis was to examine changes in cigarette smoking among the subgroup of participants reporting comorbid tobacco use in the ADAPT-2 trial.
Methods: The multi-site ADAPT-2 study used a randomized, double blind, sequential parallel comparison design to evaluate treatment with extended-release injectable NTX (380 mg every 3 weeks) combined with once-daily oral extended-release BUP (450 mg/day) vs matching injectable and oral placebo in outpatients with moderate or severe MA use disorder. The study assessed smoking outcomes, based on self-reported timeline followback (TLFB) data, twice/week for 13 weeks.
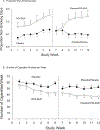
Results: Of the 403 participants in the ADAPT-2 trial, 290 reported being current cigarette smokers (71.9 %). The study found significant differences (p's < 0.0001) for each smoking outcome indicating greater change in the proportion of nonsmoking days, number of cigarettes smoked per week, and consecutive nonsmoking days, all favoring the group receiving NTX-BUP versus placebo.
Conclusions: NTX-BUP was associated with significant reductions in self-reported cigarette smoking in the context of concurrent treatment for MA use disorder. These off-target medication effects warrant prospective investigation using biochemically confirmed measures of smoking abstinence. The development of NTX-BUP as a co-addiction treatment strategy has a potential for high public health impact.
Keywords: ADAPT-2 trial; Bupropion; Co-occurring substances; Combination pharmacotherapy; Methamphetamine; Naltrexone; Tobacco use.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Madhukar Trivedi: Within the past 12 months, Dr. Trivedi has provided consulting services to Axsome Therapeutics, Biogen MA Inc., Cerebral Inc., Circular Genomics Inc, Compass Pathfinder Limited, Daiichi Sankyo Inc, GH Research Limited, Heading Health Inc, Janssen, Legion Health Inc, Merck Sharp & Dohme Corp., Mind Medicine (MindMed) Inc, Merck Sharp & Dhome LLC, Naki Health, Ltd., Neurocrine Biosciences Inc, Otsuka American Pharmaceutical Inc, Otsuka Pharmaceutical Development & Commercialization Inc, Praxis Precision Medicines Inc, Relmada Therapeutics, Inc, SAGE Therapeutics, Signant Health, Sparian Biosciences Inc, Takeda Pharmaceutical Company Ltd, and WebMD. He sits on the Scientific Advisory Board of Alto Neuroscience Inc, Cerebral Inc., Compass Pathfinder Limited, Heading Health, GreenLight VitalSign6 Inc, Legion Health Inc, and Merck Sharp & Dohme Corp. He holds stock in Alto Neuroscience Inc, Cerebral Inc, Circular Genomics Inc, GreenLight VitalSign6 Inc, Legion Health Inc. Additionally, he has received editorial compensation from American Psychiatric Association, and Oxford University Press. Dr. Thomas Carmody: Dr. Carmody has served as a consultant for Alkermes, Inc. Dr. Stephen Shoptaw: Dr. Shoptaw has received clinical supplies for this research from Alkermes, Inc.
Figures

Similar articles
-
Extended observation of reduced methamphetamine use with combined naltrexone plus bupropion in the ADAPT-2 trial.Addiction. 2024 Oct;119(10):1840-1845. doi: 10.1111/add.16529. Epub 2024 Jun 10. Addiction. 2024. PMID: 38856086 Clinical Trial.
-
Bupropion and naltrexone for smoking cessation: A double-blind randomized placebo-controlled clinical trial.Clin Pharmacol Ther. 2016 Oct;100(4):344-52. doi: 10.1002/cpt.402. Epub 2016 Jun 20. Clin Pharmacol Ther. 2016. PMID: 27213949 Free PMC article. Clinical Trial.
-
Utilizing a Two-stage Design to Investigate the Safety and Potential Efficacy of Monthly Naltrexone Plus Once-daily Bupropion as a Treatment for Methamphetamine Use Disorder.J Addict Med. 2016 Jul-Aug;10(4):236-43. doi: 10.1097/ADM.0000000000000218. J Addict Med. 2016. PMID: 27379819 Free PMC article.
-
A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations.Clin Ther. 2008 May;30(5):800-12. doi: 10.1016/j.clinthera.2008.05.010. Clin Ther. 2008. PMID: 18555928 Review.
-
Interventions for Tobacco Cessation in Adults, Including Pregnant Women: An Evidence Update for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 Jan. Report No.: 20-05264-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 Jan. Report No.: 20-05264-EF-1. PMID: 33523610 Free Books & Documents. Review.
Cited by
-
Novel potential pharmacological approaches in treating eating disorders comorbid with substance use disorders.Biomed Pharmacother. 2025 Aug;189:118327. doi: 10.1016/j.biopha.2025.118327. Epub 2025 Jul 4. Biomed Pharmacother. 2025. PMID: 40616879 Review.
-
IUPHAR Review: New strategies for medications to treat substance use disorders.Pharmacol Res. 2024 Feb;200:107078. doi: 10.1016/j.phrs.2024.107078. Epub 2024 Jan 20. Pharmacol Res. 2024. PMID: 38246477 Free PMC article. Review.
-
Potential effect of antidepressants on remission from cocaine use disorder - A nationwide matched retrospective cohort study.Drug Alcohol Depend. 2023 Oct 1;251:110958. doi: 10.1016/j.drugalcdep.2023.110958. Epub 2023 Sep 7. Drug Alcohol Depend. 2023. PMID: 37703770 Free PMC article.
References
-
- Almeida LE, Pereira EF, Alkondon M, Fawcett WP, Randall WR, & Albuquerque EX (2000). The opioid antagonist naltrexone inhibits activity and alters expression of alpha7 and alpha4beta2 nicotinic receptors in hippocampal neurons: Implications for smoking cessation programs. Neuropharmacology, 39(13), 2740–2755. 10.1016/s0028-3908(00)00157-x - DOI - PubMed
-
- Anderson AL, Li SH, Markova D, Holmes TH, Chiang N, Kahn R, & Elkashef AM (2015). Bupropion for the treatment of methamphetamine dependence in non-daily users: A randomized, double-blind, placebo-controlled trial. Drug and Alcohol Dependence, 150, 170–174. 10.1016/j.drugalcdep.2015.01.036 - DOI - PMC - PubMed

