Mutational analysis of a conserved positive charge in the c-ring of E. coli ATP synthase
- PMID: 36822493
- PMCID: PMC9998364
- DOI: 10.1016/j.bbabio.2023.148962
Mutational analysis of a conserved positive charge in the c-ring of E. coli ATP synthase
Abstract
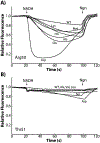
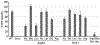
F1Fo ATP synthase is a ubiquitous molecular motor that utilizes a rotary mechanism to synthesize adenosine triphosphate (ATP), the fundamental energy currency of life. The membrane-embedded Fo motor converts the electrochemical gradient of protons into rotation, which is then used to drive the conformational changes in the soluble F1 motor that catalyze ATP synthesis. In E. coli, the Fo motor is composed of a c10 ring (rotor) alongside subunit a (stator), which together provide two aqueous half channels that facilitate proton translocation. Previous work has suggested that Arg50 and Thr51 on the cytoplasmic side of each subunit c are involved in the proton translocation process, and positive charge is conserved in this region of subunit c. To further investigate the role of these residues and the chemical requirements for activity at these positions, we generated 13 substitution mutants and assayed their in vitro ATP synthesis, H+ pumping, and passive H+ permeability activities, as well as the ability of mutants to carry out oxidative phosphorylation in vivo. While polar and hydrophobic mutations were generally tolerated in either position, introduction of negative charge or removal of polarity caused a substantial defect. We discuss the possible effects of altered electrostatics on the interaction between the rotor and stator, water structure in the aqueous channel, and interaction of the rotor with cardiolipin.
Keywords: ATP synthase; Cardiolipin; Oxidative phosphorylation; Proton transport; Site-directed mutagenesis.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

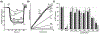
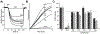



References
-
- Kühlbrandt W, Structure and Mechanisms of F-Type ATP Synthases, Annu. Rev. Biochem, 88 (2019) 515–549. - PubMed
-
- Carraro M, Checchetto V, Szabó I, Bernardi P, F- ATP synthase and the permeability transition pore: fewer doubts, more certainties, FEBS Lett, 593 (2019) 1542–1553. - PubMed
-
- Nesci S, Trombetti F, Algieri C, Pagliarani A, A Therapeutic Role for the F1FO-ATP Synthase, SLAS Discov., 24 (2019) 893–903. - PubMed
-
- Hards K, Cook GM, Targeting bacterial energetics to produce new antimicrobials, Drug Resist. Updat, 36 (2018) 1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

