Armadillo repeat only protein GS10 negatively regulates brassinosteroid signaling to control rice grain size
- PMID: 36822628
- PMCID: PMC10231457
- DOI: 10.1093/plphys/kiad117
Armadillo repeat only protein GS10 negatively regulates brassinosteroid signaling to control rice grain size
Abstract
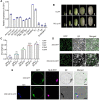
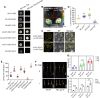
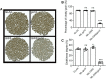
Grain yield and grain quality are major determinants in modern breeding controlled by many quantitative traits loci (QTLs) in rice (Oryza sativa). However, the mechanisms underlying grain shape and quality are poorly understood. Here, we characterize a QTL for grain size and grain quality via map-based cloning from wild rice (W1943), GS10 (Grain Size on Chromosome 10), which encodes a protein with 6 tandem armadillo repeats. The null mutant gs10 shows slender and narrow grains with altered cell size, which has a pleiotropic effect on other agronomical traits. Functional analysis reveals that GS10 interacts with TUD1 (Taihu Dwarf1) and is epistatic to OsGSK2 (glycogen synthase kinase 2) through regulating grain shape and lamina joint inclination, indicating it is negatively involved in brassinosteroid (BR) signaling. Pyramiding gs10 and the grain size gene GW5 into cultivar GLA4 substantially improved grain shape and appearance quality. Natural variation analysis revealed that gs10 from the wild rice Oryza rufipogon W1943 is a rare allele across the rice population. Collectively, these findings advance our understanding of the underlying mechanism of grain shape and provide the beneficial allele of gs10 for future rice breeding and genetic improvement.
© American Society of Plant Biologists 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

