HTR2A agonists play a therapeutic role by restricting ILC2 activation in papain-induced lung inflammation
- PMID: 36823235
- PMCID: PMC10066198
- DOI: 10.1038/s41423-023-00982-6
HTR2A agonists play a therapeutic role by restricting ILC2 activation in papain-induced lung inflammation
Abstract
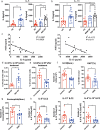
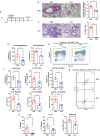
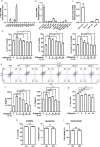
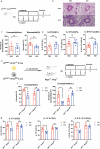
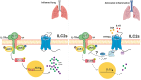
Group 2 innate lymphoid cells (ILC2s) are a category of heterogeneous cells that produce the cytokines IL-5 and IL-13, which mediate the type 2 immune response. However, specific drug targets on lung ILC2s have rarely been reported. Previous studies have shown that type 2 cytokines, such as IL-5 and IL-13, are related to depression. Here, we demonstrated the negative correlation between the depression-associated monoamine neurotransmitter serotonin and secretion of the cytokines IL-5 and IL-13 by ILC2s in individuals with depression. Interestingly, serotonin ameliorates papain-induced lung inflammation by suppressing ILC2 activation. Our data showed that the serotonin receptor HTR2A was highly expressed on ILC2s from mouse lungs and human PBMCs. Furthermore, an HTR2A selective agonist (DOI) impaired ILC2 activation and alleviated the type 2 immune response in vivo and in vitro. Mice with ILC2-specific depletion of HTR2A (Il5cre/+·Htr2aflox/flox mice) abolished the DOI-mediated inhibition of ILC2s in a papain-induced mouse model of inflammation. In conclusion, serotonin and DOI could restrict the type 2 lung immune response, indicating a potential treatment strategy for type 2 lung inflammation by targeting HTR2A on ST2+ ILC2s.
Keywords: DOI; Group 2 innate lymphoid cell; HTR2A; Serotonin (5-HT); Type 2 lung inflammation.
© 2023. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
Serotonin suppresses lung ILC2 activation and proliferation.Cell Mol Immunol. 2023 May;20(5):546-547. doi: 10.1038/s41423-023-00996-0. Epub 2023 Apr 4. Cell Mol Immunol. 2023. PMID: 37012397 Free PMC article. No abstract available.
References
-
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. - PubMed
-
- Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018;286:37–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

