Gut microbial metabolism of 5-ASA diminishes its clinical efficacy in inflammatory bowel disease
- PMID: 36823301
- PMCID: PMC10928503
- DOI: 10.1038/s41591-023-02217-7
Gut microbial metabolism of 5-ASA diminishes its clinical efficacy in inflammatory bowel disease
Abstract
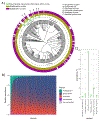
For decades, variability in clinical efficacy of the widely used inflammatory bowel disease (IBD) drug 5-aminosalicylic acid (5-ASA) has been attributed, in part, to its acetylation and inactivation by gut microbes. Identification of the responsible microbes and enzyme(s), however, has proved elusive. To uncover the source of this metabolism, we developed a multi-omics workflow combining gut microbiome metagenomics, metatranscriptomics and metabolomics from the longitudinal IBDMDB cohort of 132 controls and patients with IBD. This associated 12 previously uncharacterized microbial acetyltransferases with 5-ASA inactivation, belonging to two protein superfamilies: thiolases and acyl-CoA N-acyltransferases. In vitro characterization of representatives from both families confirmed the ability of these enzymes to acetylate 5-ASA. A cross-sectional analysis within the discovery cohort and subsequent prospective validation within the independent SPARC IBD cohort (n = 208) found three of these microbial thiolases and one acyl-CoA N-acyltransferase to be epidemiologically associated with an increased risk of treatment failure among 5-ASA users. Together, these data address a longstanding challenge in IBD management, outline a method for the discovery of previously uncharacterized gut microbial activities and advance the possibility of microbiome-based personalized medicine.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Figures

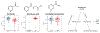


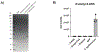

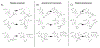






Comment in
-
Metabolism of 5-ASA by the gut microbiome.Nat Rev Gastroenterol Hepatol. 2023 May;20(5):269. doi: 10.1038/s41575-023-00777-0. Nat Rev Gastroenterol Hepatol. 2023. PMID: 37012323 No abstract available.
-
Pharmacomicrobiomics in IBD: Should We Select Treatments Based on Gut Microbes?Gastroenterology. 2023 Sep;165(3):802-803. doi: 10.1053/j.gastro.2023.04.021. Epub 2023 Apr 29. Gastroenterology. 2023. PMID: 37127097 No abstract available.
References
-
- Ford AC et al. Efficacy of 5-Aminosalicylates in Ulcerative Colitis: Systematic Review and Meta-Analysis. Official journal of the American College of Gastroenterology | ACG 106, 601–616 (2011). - PubMed
-
- Ford AC et al. Efficacy of 5-Aminosalicylates in Crohn’s Disease: Systematic Review and Meta-Analysis. Official journal of the American College of Gastroenterology | ACG 106, 617–629 (2011). - PubMed
Methods-only references:
-
- Lohman BK, Weber JN & Bolnick DI Evaluation of TagSeq, a reliable low-cost alternative for RNAseq. Molecular Ecology Resources 16, 1315–1321 (2016). - PubMed
-
- Oksanen J et al. The vegan package. Community ecology package 10, 719 (2007).
-
- Wickham H ggplot2: elegant graphics for data analysis. (springer, 2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

