Lineage tracing of mutant granulosa cells reveals in vivo protective mechanisms that prevent granulosa cell tumorigenesis
- PMID: 36823373
- PMCID: PMC10154338
- DOI: 10.1038/s41418-023-01132-1
Lineage tracing of mutant granulosa cells reveals in vivo protective mechanisms that prevent granulosa cell tumorigenesis
Abstract
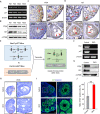
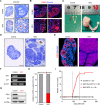
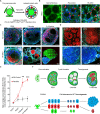
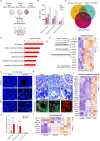
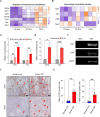
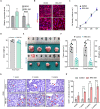
Ovarian granulosa cell tumors (GCTs) originate from granulosa cells (GCs) and represent the most common sex cord-stromal tumor in humans. However, the developmental regulations and molecular mechanisms underlying their etiology are largely unknown. In the current study, we combined a multi-fluorescent reporter mouse model with a conditional knockout mouse model, in which the tumor suppressor genes Pten and p27 were deleted in GCs, to perform cell lineage tracing of mutant GCs. We found that only 30% of ovaries with substantial mutant GCs developed into GCTs that derived from a single mutant GC. In-depth molecular analysis of the process of tumorigenesis demonstrated that up-regulation of immune evasion genes Cd24a and Cd47 led, in part, to the transition of mutant GCs to GCTs. Therefore, treatment with the Cd47 inhibitor RRX-001 was tested and found to efficiently suppress the growth of GCTs in vivo. Together, our study has revealed an immune evasion mechanism via CD24/CD47 upregulation to GCT formation, shedding light on the future potential clinical therapies for GCTs.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Albertini DF, Barrett SL. Oocyte-somatic cell communication. Reprod Suppl. 2003;61:49–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

