Intranasal multivalent adenoviral-vectored vaccine protects against replicating and dormant M.tb in conventional and humanized mice
- PMID: 36823425
- PMCID: PMC9948798
- DOI: 10.1038/s41541-023-00623-z
Intranasal multivalent adenoviral-vectored vaccine protects against replicating and dormant M.tb in conventional and humanized mice
Abstract
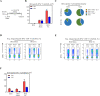
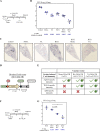
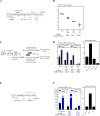
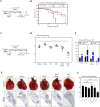
Viral-vectored vaccines are highly amenable for respiratory mucosal delivery as a means of inducing much-needed mucosal immunity at the point of pathogen entry. Unfortunately, current monovalent viral-vectored tuberculosis (TB) vaccine candidates have failed to demonstrate satisfactory clinical protective efficacy. As such, there is a need to develop next-generation viral-vectored TB vaccine strategies which incorporate both vaccine antigen design and delivery route. In this study, we have developed a trivalent chimpanzee adenoviral-vectored vaccine to provide protective immunity against pulmonary TB through targeting antigens linked to the three different growth phases (acute/chronic/dormancy) of Mycobacterium tuberculosis (M.tb) by expressing an acute replication-associated antigen, Ag85A, a chronically expressed virulence-associated antigen, TB10.4, and a dormancy/resuscitation-associated antigen, RpfB. Single-dose respiratory mucosal immunization with our trivalent vaccine induced robust, sustained tissue-resident multifunctional CD4+ and CD8+ T-cell responses within the lung tissues and airways, which were further quantitatively and qualitatively improved following boosting of subcutaneously BCG-primed hosts. Prophylactic and therapeutic immunization with this multivalent trivalent vaccine in conventional BALB/c mice provided significant protection against not only actively replicating M.tb bacilli but also dormant, non-replicating persisters. Importantly, when used as a booster, it also provided marked protection in the highly susceptible C3HeB/FeJ mice, and a single respiratory mucosal inoculation was capable of significant protection in a humanized mouse model. Our findings indicate the great potential of this next-generation TB vaccine strategy and support its further clinical development for both prophylactic and therapeutic applications.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- WHO. W.H.O. Global Tuberculosis Report 2021 (WHO, 2021).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

