Efficiency of cutaneous heat diffusion after local hyperthermia for the treatment of itch
- PMID: 36823504
- PMCID: PMC10155804
- DOI: 10.1111/srt.13277
Efficiency of cutaneous heat diffusion after local hyperthermia for the treatment of itch
Abstract
Background: Today, itching is understood as an independent sensory perception, which is based on a complex etiology of a disturbed neuronal activity and leads to clinical symptoms. The primary afferents (pruriceptors) have functional overlaps with afferents of thermoregulation (thermoceptors). Thus, an antipruritic effect can be caused by antagonizing heat-sensitive receptors of the skin. The ion channel TRP-subfamily V member 1 (TRPV1) is of particular importance in this context. Repeated heat application can induce irreversible inactivation by unfolding of the protein, causing a persistent functional deficit and thus clinically and therapeutically reducing itch sensation.
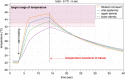
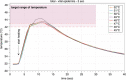
Material and methods: To demonstrate relevant heat diffusion after local application of heat (45°C to 52°C for 3 and 5 seconds) by a technical medical device, the temperature profile for the relevant skin layer was recorded synchronously on ex vivo human skin using an infrared microscope.
Results: The results showed that the necessary activation temperature for TRPV1 of (≥43°C) in the upper relevant skin layers was safely reached after 3 and 5 seconds of application time. There were no indications of undesirable thermal effects.
Conclusion: The test results show that the objectified performance of the investigated medical device can be expected to provide the necessary temperature input for the activation of heat-sensitive receptors in the skin. Clinical studies are necessary to prove therapeutic efficacy in the indication pruritus.
Keywords: TRPV1; concentrated heat; itch; local hyperthermia; pruritus.
© 2023 The Authors. Skin Research and Technology published by John Wiley & Sons Ltd.
Conflict of interest statement
Johannes Wohlrab has received fees for lecturing and/or consulting, and/or received funding for scientific projects and/or clinical studies from Abbvie, ACA, Actelion, Allergika, Almirall, Agfa, Aristo, Astellas, BayPharma, Baxalta, Beiersdorf, BMS, Biogen, Boehringer Ingelheim, Bombastus, Celgene, Dermapharm, Ei, Evolva, Evonik, Galderma, Grünenthal, GSK, Hexal, Infectopharm, Janssen‐Cilag, Jenapharm, Johnson & Johnson, Klinge, Klosterfrau, Leo, Lilly, L‘Oréal, Mavena, Medac, Medice, Mibe, MSD, Mylan, Novaliq, Novartis, Pfizer, Pohl‐Boskamp, Riemser, Sanofi, Skinomics, Wolff. Tim Mentel is an employee of Mibetec GmbH. Adina Eichner declares no conflict of interest. The investigations have been fully funded by Mibetec GmbH (Sandersdorf‐Brehna, Germany).
Figures




Similar articles
-
Deletion of the murine ATP/UTP receptor P2Y2 alters mechanical and thermal response properties in polymodal cutaneous afferents.Neuroscience. 2016 Sep 22;332:223-30. doi: 10.1016/j.neuroscience.2016.06.054. Epub 2016 Jul 5. Neuroscience. 2016. PMID: 27393251 Free PMC article.
-
Transient Receptor Potential Channels and Itch.Int J Mol Sci. 2022 Dec 27;24(1):420. doi: 10.3390/ijms24010420. Int J Mol Sci. 2022. PMID: 36613861 Free PMC article. Review.
-
TRP channels as novel players in the pathogenesis and therapy of itch.Biochim Biophys Acta. 2007 Aug;1772(8):1004-21. doi: 10.1016/j.bbadis.2007.03.002. Epub 2007 Mar 19. Biochim Biophys Acta. 2007. PMID: 17462867 Review.
-
TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity.Mol Pain. 2013 Mar 4;9:9. doi: 10.1186/1744-8069-9-9. Mol Pain. 2013. PMID: 23497345 Free PMC article.
-
The role of TRPV1 channel in aged human skin.J Dermatol Sci. 2012 Feb;65(2):81-5. doi: 10.1016/j.jdermsci.2011.11.003. Epub 2011 Nov 18. J Dermatol Sci. 2012. PMID: 22154816 Review.
References
-
- Yosipovitch G, Misery L, Proksch E, Metz M, Stander S, Schmelz M. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99(13):1201‐1209. - PubMed
-
- Misery L, Weisshaar E, Brenaut E, et al. Pathophysiology and management of sensitive skin: position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch (IFSI). J Eur Acad Dermatol Venereol. 2020;34(2):222‐229. - PubMed
-
- Najafi P, Carre JL, Ben Salem D, Brenaut E, Misery L, Dufor O. Central mechanisms of itch: a systematic literature review and meta‐analysis. J Neuroradiol. 2020;47(6):450‐457. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

