Strategic discussion on funding and access to therapies targeting rare diseases in Spain: an expert consensus paper
- PMID: 36823598
- PMCID: PMC9950008
- DOI: 10.1186/s13023-023-02635-3
Strategic discussion on funding and access to therapies targeting rare diseases in Spain: an expert consensus paper
Abstract
Background: In recent years, significant advances have been made in the field of rare diseases (RDs). However, there is a large number of RDs without specific treatment and half of these treatments have public funding in Spain. The aim of the FINEERR project was to carry out a multidisciplinary strategic discussion on the challenge of funding and access to RD-targeted drugs in Spain, in order to agree on specific proposals for medium-term improvement and hence support decision-making in the Spanish National Healthcare System (SNHS).
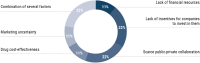
Results: The FINEERR Project was organized around a CORE Advisory Committee, which provided an overview, agreed on the design and scope of the project, and selected the members within each of four working groups (WG). Overall, 40 experts discussed and reached a consensus on different relevant aspects, such as conditioning factors for initial funding and access, evaluation and access to RD-targeted therapies, funding of these therapies, and implementation of a new funding and access model. From these meetings, 50 proposals were defined and classified by their level of relevance according to the experts. A descriptive analysis of responses was performed for each proposal. Thereafter, experts completed another questionnaire where they ranked the 25 most relevant proposals according to their level of feasibility of being implemented in the SNHS. The most relevant and feasible proposals were to improve: process of referral of patients with RDs, control over monitoring mechanisms, and communication between healthcare professionals and patients.
Conclusions: The FINEERR project may provide a starting point for stakeholders involved in the process of funding and access to RD-targeted therapies in Spain to provide the necessary resources and implement measures to improve both the quality of life and life expectancy of patients with RDs.
Keywords: Access; Funding; Measures; Orphan drugs; Rare diseases; Reflection; Regulatory science; Reimbursement; Spain.
© 2023. The Author(s).
Conflict of interest statement
NZ, JV, FA, and AHV work at Weber, a consulting company which has received funding from Roche Farma S.A. (Spain) for the development of this project. AA, IM, MTB, OD, and PF received fees from Weber for their participation in this project. The authors declare to have no financial or proprietary interests in any material discussed in this article.
Figures




References
-
- Chan AYL, Chan VKY, Olsson S, Fan M, Jit M, Gong M, et al. Orphan drugs—access and unmet needs in 194 countries and six regions: a comprehensive policy review with content analysis. The Lancet. 2019;1(394):S72. doi: 10.1016/S0140-6736(19)32408-0. - DOI
-
- Comisión Europea. Evaluación conjunta del Reglamento (CE) no 1901/2006 del Parlamento Europeo y del Consejo, de 12 de diciembre de 2006, sobre medicamentos para uso pediátrico, y del Reglamento (CE) no 141/2000 del Parlamento Europeo y del Consejo, de 16 de diciembre de 1999, sobre medicamentos huérfanos. 2020. https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/or.... Accessed 22 Jun 2022.
-
- OCDE. Pharmaceutical reimbursement and pricing in Germany. 2018. https://www.oecd.org/els/health-systems/Pharmaceutical-Reimbursement-and.... Accessed 19 May 2022.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

