IL-6 as new prognostic factor in patients with advanced cutaneous squamous cell carcinoma treated with cemiplimab
- PMID: 36823670
- PMCID: PMC9948392
- DOI: 10.1186/s12967-023-03971-5
IL-6 as new prognostic factor in patients with advanced cutaneous squamous cell carcinoma treated with cemiplimab
Abstract
Background: Prognostic factors for initial response of advanced cutaneous squamous cell carcinoma to cemiplimab treatment are lacking. Il-6 has been found to affect immune cell populations which impact tumor development. The aim was to investigate the prognostic significance of IL-6 serum levels before and during treatment.
Methods: Serum levels of IL-6 were correlated with clinical outcomes in a retrospective study.
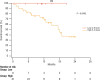
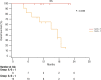
Results: Overall, 39 patients were enrolled. High serum levels of IL-6 (> 5.6 pg/ml) were associated with poorer survival (45.1% vs 0 deaths; OS: 16.1 ± 1.5 vs 20.8 ± 0 months, 95% CI 13,046 to 19,184) and shorter PFS (10.3 ± 1.9 vs 18.9 ± 1.5 months; 95% CI 3433 to 10,133) in patients with advanced CSCC treated with cemiplimab. In addition, patients whose IL-6 level increased after treatment with cemiplimab, independently of the basal level, had a poorer response to treatment than patients whose level was reduced or stable after immunotherapy.
Conclusions: Serum levels of IL-6 at baseline and changes after cemiplimab immunotherapy may have a prognostic significance in patients with advanced cutaneous squamous cell carcinoma.
Keywords: Cemiplimab; Cutaneous squamous cell carcinoma; IL-6; Prognostic factor.
© 2023. The Author(s).
Conflict of interest statement
PAA has/had a consultant/advisory role for Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-Al Health. He also received research funding from Bristol Myers Squibb, Roche-Genentech, Pfizer, Sanofi. AC received grant consultancies from BMS, Astrazeneca, Roche and MSD. He also received speaker's fee from Astrazeneca, Novartis and Eisai.
Figures



References
-
- Argenziano G, Fargnoli MC, Fantini F, Gattoni M, Gualdi G, Pastore F, et al. Identifying candidates for immunotherapy with cemiplimab to treat advanced cutaneous squamous cell carcinoma: an expert opinion. Ther Adv Med Oncol. 2022;14:17588359211066272. doi: 10.1177/17588359211066272. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

