Implantable Shock Absorber Provides Superior Pain Relief and Functional Improvement Compared With High Tibial Osteotomy in Patients with Mild-to-Moderate Medial Knee Osteoarthritis: A 2-Year Report
- PMID: 36823955
- PMCID: PMC10416201
- DOI: 10.1177/19476035231157335
Implantable Shock Absorber Provides Superior Pain Relief and Functional Improvement Compared With High Tibial Osteotomy in Patients with Mild-to-Moderate Medial Knee Osteoarthritis: A 2-Year Report
Abstract
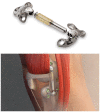
Objective: Up to 10 million Americans below the age of 65 years have symptomatic knee osteoarthritis (OA) and may not yet be candidates for arthroplasty. In response, a subcutaneous implantable shock absorber (ISA) that unloads the knee has been developed. The safety and effectiveness of ISA treatment were compared against a surgical unloading control, high tibial osteotomy (HTO).
Design: This was a prospective open-label cohort study with a historical control arm. Subjects underwent ISA placement or HTO. The primary endpoint was a composite variable combining pain, function, specific adverse events, integrity of implant or hardware, and conversion to subsequent surgery. Pain and function outcomes (Western Ontario and McMaster Universities Arthritis Index scores) were assessed through 24 months. Adverse events were tracked.
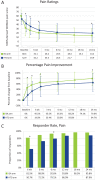
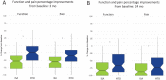
Results: The primary endpoint demonstrated superiority of the ISA arm versus the HTO arm, with 85.6% of ISA subjects meeting all criteria compared with 65.5% of HTO subjects. In addition, all 5 secondary endpoints showed superiority of ISA over HTO. At 24 months, the proportions of subjects considered responders were 95.8% (ISA) versus 87.9% (HTO) for pain and 91.7% (ISA) versus 81.3% (HTO) for function. The ISA procedure was well tolerated, with 13.4 days to full weightbearing status versus 58.0 days for the HTO arm.
Conclusions: Treatment with an ISA demonstrated noninferiority and superiority versus treatment with HTO in subjects aged 25-65 years who had OA of the medial knee. Treatment with ISA has high clinical benefit and is durable through at least 24 months.
Trial registration: ClinicalTrials.gov NCT03671213 NCT03838978.
Keywords: high tibial osteotomy; knee; osteoarthritis; shock absorber; unloading.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors received institutional support from Moximed regarding this study. In addition, DRD is a consultant for Depuy Synthes and Osteocentric, has received royalties from Smith and Nephew, and has received institutional research support from Zimmer, Aesculap, and Donjoy. ASR is a consultant for Moximed, Enhatch, Conformis, Smith & Nephew, Anika, Bodycad, Xiros, NewClip, Ranfac, Marrow Cellution, and Cervos.
Figures



References
-
- Centers for Disease Control and Prevention (CDC). Arthritis in America. CDC. Published November 8, 2018. Accessed June 2,2022. https://www.cdc.gov/vitalsigns/arthritis/index.html.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

