Oxygenic photosynthetic responses of cyanobacteria exposed under an M-dwarf starlight simulator: Implications for exoplanet's habitability
- PMID: 36824196
- PMCID: PMC9941696
- DOI: 10.3389/fpls.2023.1070359
Oxygenic photosynthetic responses of cyanobacteria exposed under an M-dwarf starlight simulator: Implications for exoplanet's habitability
Abstract
Introduction: The search for life on distant exoplanets is expected to rely on atmospheric biosignatures detection, such as oxygen of biological origin. However, it is not demonstrated how much oxygenic photosynthesis, which on Earth depends on visible light, could work under spectral conditions simulating exoplanets orbiting the Habitable Zone of M-dwarf stars, which have low light emission in the visible and high light emission in the far-red/near-infrared. By utilizing cyanobacteria, the first organisms to evolve oxygenic photosynthesis on our planet, and a starlight simulator capable of accurately reproducing the emission spectrum of an M-dwarf in the range 350-900 nm, we could answer this question.
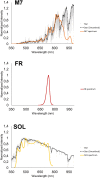
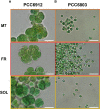
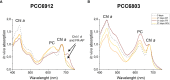
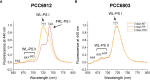
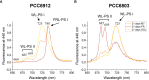
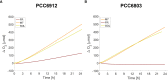
Methods: We performed experiments with the cyanobacterium Chlorogloeopsis fritschii PCC6912, capable of Far-Red Light Photoacclimation (FaRLiP), which allows the strain to harvest far-red in addition to visible light for photosynthesis, and Synechocystis sp. PCC6803, a species unable to perform this photoacclimation, comparing their responses when exposed to three simulated light spectra: M-dwarf, solar and far-red. We analysed growth and photosynthetic acclimation features in terms of pigment composition and photosystems organization. Finally, we determined the oxygen production of the strains directly exposed to the different spectra.
Results: Both cyanobacteria were shown to grow and photosynthesize similarly under M-dwarf and solar light conditions: Synechocystis sp. by utilizing the few photons in the visible, C. fritschii by harvesting both visible and far-red light, activating the FaRLiP response.
Discussion: Our results experimentally show that an M-dwarf light spectrum could support a biological oxygen production similar to that in solar light at the tested light intensities, suggesting the possibility to discover such atmospheric biosignatures on those exoplanets if other boundary conditions are met.
Keywords: M-dwarf spectrum; biosignatures; cyanobacteria; laboratory simulations; light acclimation; oxygenic photosynthesis.
Copyright © 2023 Battistuzzi, Cocola, Claudi, Pozzer, Segalla, Simionato, Morosinotto, Poletto and La Rocca.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Adams F. C., Laughlin G. (1997). A dying universe: The long-term fate and evolution of astrophysical objects. Rev. Modern Phys. 69 (2), 337. doi: 10.1103/revmodphys.69.337 - DOI
-
- Barnes R. (2017). Tidal locking of habitable exoplanets. Celestial Mechanics Dynamical Astronomy 129 (4), 509–536. doi: 10.1007/s10569-017-9783-7 - DOI
-
- Battistuzzi M., Cocola L., Salasnich B., Erculiani M. S, Alei E., Morosinotto T., et al. . (2020). A new remote sensing-based system for the monitoring and analysis of growth and gas exchange rates of photosynthetic microorganisms under simulated non-terrestrial conditions. Front. Plant Sci. 11 (March). doi: 10.3389/fpls.2020.00182 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

