Curcumin-QingDai Combination as Treatment for Moderate-Severe Ulcerative Colitis
- PMID: 36824698
- PMCID: PMC9941783
- DOI: 10.1159/000526646
Curcumin-QingDai Combination as Treatment for Moderate-Severe Ulcerative Colitis
Abstract
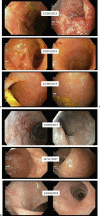
Curcumin was shown in placebo-controlled trials to induce remission in mild-moderate ulcerative colitis (UC). QingDai (QD, Indigo), another herbal extract, showed efficacy in two UC trials from Japan, but evidence in the Western population is scant. We report on the use of curcumin-QingDai combination (CurQD) for the treatment of moderate-severe UC. Patient 1 was a 24-year-old male with severe UC refractory to cyclosporine and corticosteroids. He partially responded to infliximab but later lost response to an optimized dose of infliximab in combination with 6-mercaptopurine, presenting with worsening symptoms and severe Mayo 3 mucosal inflammation. Initiation of CurQD 2.5 g/day resulted in rapid cessation of blood per rectum. Complete clinical remission ensued within few weeks. Follow-up endoscopies performed 12 weeks later showed only minimal residual inflammation. Infliximab was later stopped due to reimbursement issues, and the patient was successfully maintained on lower doses of CurQD and 6-mercaptopurine for 31 months. Two flares have responded to a temporary increase in QD component dose. Patient 2 was a 59-year-old female with extensive UC not responding to maximal oral + topical 5-ASA and corticosteroids. Despite severe mucosal ulceration (Mayo 3) found on endoscopy, she refused the recommendation for biologics and opted for a short-term limited trial of CurQD. This was initiated at 2,000 mg/day and induced rapid clinical remission. Lower endoscopies performed after 2 and 5 months on CurQD showed complete mucosal healing, and the patient maintained her clinical remission on low-dose CurQD for 49 months. No adverse events were noted in the 2 patients.
Keywords: Inflammatory bowel disease; Treatment; Ulcerative colitis.
Copyright © 2022 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Shomron Ben-Horin has received advisory board and/or consulting fees from Abbvie, Takeda, Janssen, Celltrion, Pfizer, GSK, Ferring, Novartis, Roche, Gilead, NeoPharm, Predicta Med, Galmed, Medial Earlysign, and Eli Lilly and research support from Abbvie, Takeda, Janssen, Celltrion, Pfizer, and Galmed. The Chaim Sheba Medical Center has filed intellectual property requests on the combination of curcumin and QD. EviNature is a spin-off company of Sheba Medical Center; Shomron Ben-Horin and Nir Salomon are employed by and hold equity in EviNature. The UK has received speaker fees from Abbvie, Janssen, BMS, Rafa, Novartis, Pfizer, and Takeda; research support from Takeda and Janssen; and consulting fees from Takeda and CTS.
Figures
References
-
- Lang A, Salomon N, Wu JCY, Kopylov U, Lahat A, Har-Noy O, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13((8)):1444–9.e1. - PubMed
-
- Naganuma M, Sugimoto S, Mitsuyama K, Kobayashi T, Yoshimura N, Ohi H, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018;154((4)):935–947. - PubMed
-
- Banerjee R, Pal P, Penmetsa A, Kathi P, Girish G, Goren I, et al. Novel bioenhanced curcumin with mesalamine for induction of clinical and endoscopic remission in mild-to-moderate ulcerative colitis: a randomized double-blind placebo-controlled pilot study. J Clin Gastroenterol. 2021;55((8)):702–708. - PubMed
Publication types
LinkOut - more resources
Full Text Sources