This is a preprint.
Priming locomotor training with transspinal stimulation in people with spinal cord injury: study protocol of a randomized clinical trial
- PMID: 36824823
- PMCID: PMC9949167
- DOI: 10.21203/rs.3.rs-2527617/v1
Priming locomotor training with transspinal stimulation in people with spinal cord injury: study protocol of a randomized clinical trial
Update in
-
Priming locomotor training with transspinal stimulation in people with spinal cord injury: study protocol of a randomized clinical trial.Trials. 2023 Feb 25;24(1):145. doi: 10.1186/s13063-023-07193-4. Trials. 2023. PMID: 36841773 Free PMC article.
Abstract
Background: The seemingly simple tasks of standing and walking require continuous integration of complex spinal reflex circuits between descending motor commands and ascending sensory inputs. Spinal cord injury greatly impairs standing and walking ability, but both improve with locomotor training. However, even after multiple locomotor training sessions, abnormal muscle activity and coordination persist. Thus, locomotor training alone cannot fully optimize the neuronal plasticity required to strengthen the synapses connecting the brain, spinal cord, and local circuits and potentiate neuronal activity based on need. Transcutaneous spinal cord (transspinal) stimulation alters motoneuron excitability over multiple segments by bringing motoneurons closer to threshold, a prerequisite for effectively promoting spinal locomotor network neuromodulation and strengthening neural connectivity of the injured human spinal cord. Importantly, whether concurrent treatment with transspinal stimulation and locomotor training maximizes motor recovery after spinal cord injury is unknown.
Methods: Forty-five individuals with chronic spinal cord injury are receiving 40 sessions of robotic gait training primed with 30 Hz transspinal stimulation at the Thoracic 10 vertebral level. Participants are randomized to receive 30-minutes of active or sham transspinal stimulation during standing or active transspinal stimulation while supine followed by 30-minutes of robotic gait training. Over the course of locomotor training, the body weight support, treadmill speed, and leg guidance force are adjusted as needed for each participant based on absence of knee buckling during the stance phase and toe dragging during the swing phase. At baseline and after completion of all therapeutic sessions, neurophysiological recordings registering corticospinal and spinal neural excitability changes along with clinical assessment measures of standing and walking, and autonomic function via questionnaires regarding bowel, bladder and sexual function are taken.
Discussion: The results of this mechanistic randomized clinical trial will demonstrate that tonic transspinal stimulation strengthens corticomotoneuronal connectivity and dynamic neuromodulation through posture-dependent corticospinal and spinal neuroplasticity. We anticipate that this mechanistic clinical trial will greatly impact clinical practice because in real-world clinical settings, noninvasive transspinal stimulation can be more easily and widely implemented than invasive epidural stimulation. Additionally, by applying multiple interventions to accelerate motor recovery, we are employing a treatment regimen that reflects a true clinical approach.
Trial registration: ClinicalTrials.gov: NCT04807764; Registered on March 19, 2021.
Keywords: combined interventions; locomotor training; neurophysiology; rehabilitation; spinal cord injury; standing; stepping; transspinal stimulation.
Conflict of interest statement
Competing interests {28} The authors declare that they have no competing interests.
Figures
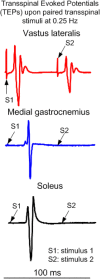
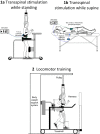
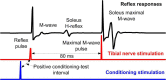

Similar articles
-
Priming locomotor training with transspinal stimulation in people with spinal cord injury: study protocol of a randomized clinical trial.Trials. 2023 Feb 25;24(1):145. doi: 10.1186/s13063-023-07193-4. Trials. 2023. PMID: 36841773 Free PMC article.
-
Brain and spinal cord paired stimulation coupled with locomotor training facilitates motor output in human spinal cord injury.Front Neurol. 2022 Oct 13;13:1000940. doi: 10.3389/fneur.2022.1000940. eCollection 2022. Front Neurol. 2022. PMID: 36313489 Free PMC article.
-
Neurophysiological Changes After Paired Brain and Spinal Cord Stimulation Coupled With Locomotor Training in Human Spinal Cord Injury.Front Neurol. 2021 May 10;12:627975. doi: 10.3389/fneur.2021.627975. eCollection 2021. Front Neurol. 2021. PMID: 34040572 Free PMC article.
-
The physiological basis of neurorehabilitation--locomotor training after spinal cord injury.J Neuroeng Rehabil. 2013 Jan 21;10:5. doi: 10.1186/1743-0003-10-5. J Neuroeng Rehabil. 2013. PMID: 23336934 Free PMC article. Review.
-
Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury.J Neurol Phys Ther. 2020 Jan;44(1):49-100. doi: 10.1097/NPT.0000000000000303. J Neurol Phys Ther. 2020. PMID: 31834165 Review.
References
-
- National Spinal Cord Injury Statistical Center, facts and figures at a glance. University of Alabama at Birmingham; 2018.
-
- van Middendorp JJ, Hosman AJF, Pouw MH, EM-SCI Study Group, Van de Meent H. ASIA impairment scale conversion in traumatic SCI: is it related with the ability to walk? A descriptive comparison with functional ambulation outcome measures in 273 patients. Spinal Cord. 2009;47:555–60. - PubMed
-
- Ellaway PH, Catley M, Davey NJ, Kuppuswamy A, Strutton P, Frankel HL, et al. Review of physiological motor outcome measures in spinal cord injury using transcranial magnetic stimulation and spinal reflexes. J Rehabil Res Dev. 2007;44:69–76. - PubMed
-
- Knikou M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin Neurophysiol. 2010;121:1655–68. - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

