This is a preprint.
Pro-phagocytic function and structural basis of GPR84 signaling
- PMID: 36824923
- PMCID: PMC9949259
- DOI: 10.21203/rs.3.rs-2535247/v1
Pro-phagocytic function and structural basis of GPR84 signaling
Update in
-
Pro-phagocytic function and structural basis of GPR84 signaling.Nat Commun. 2023 Sep 14;14(1):5706. doi: 10.1038/s41467-023-41201-0. Nat Commun. 2023. PMID: 37709767 Free PMC article.
Abstract
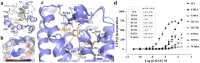
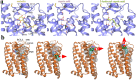
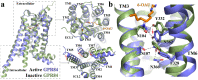
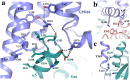
GPR84 is a unique orphan G protein-coupled receptor (GPCR) that can be activated by endogenous medium-chain fatty acids (MCFAs). The signaling of GPR84 is largely pro-inflammatory, which can augment inflammatory response, and GPR84 also functions as a pro-phagocytic receptor to enhance the phagocytic activities of macrophages. In this study, we first showed that the activation of GPR84 by the synthetic agonist 6-OAU could synergize with the blockade of CD47 on cancer cells to induce phagocytosis of cancer cells by macrophages. Then, we determined a high-resolution structure of the GPR84-Gi signaling complex with 6-OAU. This structure revealed a completely occluded binding pocket for 6-OAU, the molecular basis of receptor activation involving non-conserved structural motifs of GPR84, and an unusual Gi-coupling interface. Together with computational docking and simulations studies, our structure also suggested the mechanism for the high selectivity of GPR84 for MCFAs and the potential routes of ligand binding and dissociation. Our results provide a framework for understanding GPR84 signaling and developing new drugs targeting GPR84.
Conflict of interest statement
DECLARATION OF INTERESTS
No conflicts of interests are declared for all authors.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

