Floating Liquid Droplets on the Surface of Cryogenic Liquids: Implications for Titan Rain
- PMID: 36824999
- PMCID: PMC9940721
- DOI: 10.1021/acsearthspacechem.2c00311
Floating Liquid Droplets on the Surface of Cryogenic Liquids: Implications for Titan Rain
Abstract
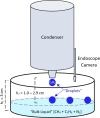
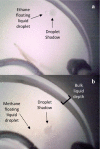
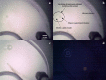
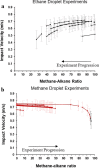
Saturn's moon, Titan, has a hydrocarbon-based hydrologic cycle with methane and ethane rainfall. Because of Titan's low gravity, "floating liquid droplets" (coherent droplets of liquid hydrocarbons that float upon a liquid surface) may form on the surface of Titan's hydrocarbon lakes and seas during rainfall. Floating liquid droplets, however, have not been investigated in the laboratory under conditions appropriate for the surface of Titan (cryogenic, hydrocarbon, liquids). We conducted a set of experiments to simulate methane and ethane rainfall under Titan surface conditions (89-94 K, 1.5 bar nitrogen atmosphere) and find that floating ethane droplets form in a wide range of bulk liquid compositions, yet floating methane droplets only form in a narrow compositional range and impact velocity. We find droplet formation is independent of the liquid density and hypothesize that dissolved atmospheric nitrogen in the bulk liquid may repel liquid ethane droplets at the surface. We propose that liquid droplets will form in Titan's methane-rich lakes and seas during ethane rainfall with a droplet radius of ≤3 mm and an impact velocity of ≤0.7 m/s. The presence of these droplets on Titan's lakes may result in a liquid surface layer that is dominated in rainfall composition.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Rayleigh L. On the Stability, or Instability, of certain Fluid Motions. Proc. London Math. Soc. 1879, s1–11, 57–72. 10.1112/plms/s1-11.1.57. - DOI
-
- Reynolds O.On the floating of drops on the surface of water depending only on the purity of the surface’ Manchester Literary and Philosophical Society, Proceedings 1881; Vol. 21.
-
- Khan & Garnier . Mechanism of Non-Coalescence for Liquid Droplets at the Air-Liquid Interface. InConference: Chemeca 2007, Melbourne, Australia, 2007.
-
- Kavehpour H. P. Coalescence of Drops. Annu. Rev. Fluid Mech. 2015, 47, 245–268. 10.1146/annurev-fluid-010814-014720. - DOI
-
- Seth J. B.; Anand C.; Das Mahajan L. XXVII Liquid drops on the same liquid surface. London, Edinburgh, Dublin Philos. Mag., J. Sci. 1929, 7 (42), 247–253. 10.1080/14786440208564736. - DOI
LinkOut - more resources
Full Text Sources
