Virulence potential of multidrug-resistant Acinetobacter baumannii isolates from COVID-19 patients on mechanical ventilation: The first report from Serbia
- PMID: 36825087
- PMCID: PMC9941878
- DOI: 10.3389/fmicb.2023.1094184
Virulence potential of multidrug-resistant Acinetobacter baumannii isolates from COVID-19 patients on mechanical ventilation: The first report from Serbia
Abstract
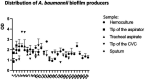
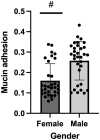
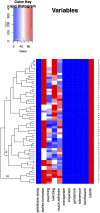
Since the WHO declared the COVID-19 pandemic in March 2020, the disease has spread rapidly leading to overload of the health system and many of the patients infected with SARS-CoV-2 needed to be admitted to the intensive care unit (ICU). Around 10% of patients with the severe manifestation of COVID-19 need noninvasive or invasive mechanical ventilation, which represent a risk factor for Acinetobacter baumannii superinfection. The 64 A. baumannii isolates were recovered from COVID-19 patients admitted to ICU at General Hospital "Dr Laza K. Lazarević" Šabac, Serbia, during the period from December 2020 to February 2021. All patients required mechanical ventilation and mortality rate was 100%. The goal of this study was to evaluate antibiotic resistance profiles and virulence potential of A. baumannii isolates recovered from patients with severe form of COVID-19 who had a need for mechanical ventilation. All tested A. baumannii isolates (n = 64) were sensitive to colistin, while resistant to meropenem, imipenem, gentamicin, tobramycin, and levofloxacin according to the broth microdilution method and MDR phenotype was confirmed. In all tested isolates, representatives of international clone 2 (IC2) classified by multiplex PCR for clonal lineage identification, bla AmpC, bla OXA-51, and bla OXA-23 genes were present, as well as ISAba1 insertion sequence upstream of bla OXA-23. Clonal distribution of one dominant strain was found, but individual strains showed phenotypic differences in the level of antibiotic resistance, biofilm formation, and binding to mucin and motility. According to PFGE, four isolates were sequenced and antibiotic resistance genes as well as virulence factors genes were analyzed in these genomes. The results of this study represent the first report on virulence potential of MDR A. baumannii from hospital in Serbia.
Keywords: AMR; Acinetobacter baumannii; COVID-19; ICU; virulence potential.
Copyright © 2023 Novović, Kuzmanović Nedeljković, Poledica, Nikolić, Grujić, Jovčić, Kojić and Filipić.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Clusters of imipenem-resistant Acinetobacter baumannii clones producing different carbapenemases in an intensive care unit.Clin Microbiol Infect. 2008 Jun;14(6):588-94. doi: 10.1111/j.1469-0691.2008.01996.x. Epub 2008 Apr 5. Clin Microbiol Infect. 2008. PMID: 18397334
-
The emergence of a novel sequence type of MDR Acinetobacter baumannii from the intensive care unit of an Egyptian tertiary care hospital.Ann Clin Microbiol Antimicrob. 2017 May 10;16(1):34. doi: 10.1186/s12941-017-0208-y. Ann Clin Microbiol Antimicrob. 2017. PMID: 28486994 Free PMC article.
-
[Investigation of oxacillinase genes in nosocomial multidrug-resistant Acinetobacter baumannii isolates by multiplex PCR and evaluation of their clonal relationship with Rep-PCR].Mikrobiyol Bul. 2015 Apr;49(2):249-58. doi: 10.5578/mb.8884. Mikrobiyol Bul. 2015. PMID: 26167825 Turkish.
-
Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit.J Antimicrob Chemother. 2006 Mar;57(3):557-61. doi: 10.1093/jac/dkl004. Epub 2006 Jan 23. J Antimicrob Chemother. 2006. PMID: 16431857
-
Phenotypic and Genotypic Characterization of Clinical Isolates Belonging to the Acinetobacter calcoaceticus-Acinetobacter baumannii (ACB) Complex Isolated From Animals Treated at a Veterinary Hospital in Switzerland.Front Vet Sci. 2019 Feb 5;6:17. doi: 10.3389/fvets.2019.00017. eCollection 2019. Front Vet Sci. 2019. PMID: 30805352 Free PMC article.
Cited by
-
Phenotypical and Molecular Characterization of Acinetobacter baumannii Isolated from Hospitalized Patients During the COVID-19 Pandemic in Brazil.Life (Basel). 2025 Apr 8;15(4):623. doi: 10.3390/life15040623. Life (Basel). 2025. PMID: 40283177 Free PMC article.
-
Acinetobacter baumannii infection in critically ill patients with COVID-19 from Tehran, Iran: the prevalence, antimicrobial resistance patterns and molecular characteristics of isolates.Front Cell Infect Microbiol. 2025 Jan 30;14:1511122. doi: 10.3389/fcimb.2024.1511122. eCollection 2024. Front Cell Infect Microbiol. 2025. PMID: 39958989 Free PMC article.
-
Bacterial Infections in Intensive Care Units: Epidemiological and Microbiological Aspects.Antibiotics (Basel). 2024 Mar 5;13(3):238. doi: 10.3390/antibiotics13030238. Antibiotics (Basel). 2024. PMID: 38534673 Free PMC article. Review.
-
Exploring Biofilm-Related Traits and Bile Salt Efficacy as Anti-Biofilm Agents in MDR Acinetobacter baumannii.Antibiotics (Basel). 2024 Sep 13;13(9):880. doi: 10.3390/antibiotics13090880. Antibiotics (Basel). 2024. PMID: 39335053 Free PMC article.
-
Geographical mapping and temporal trends of Acinetobacter baumannii carbapenem resistance: A comprehensive meta-analysis.PLoS One. 2024 Dec 16;19(12):e0311124. doi: 10.1371/journal.pone.0311124. eCollection 2024. PLoS One. 2024. PMID: 39680587 Free PMC article.
References
-
- Abdollahi A., Aliramezani A., Salehi M., Norouzi Shadehi M., Ghourchian S., Douraghi M. (2021). Co-infection of ST2IP carbapenem-resistant Acinetobacter baumannii with SARS-CoV-2 in the patients admitted to a Tehran tertiary referral hospital. BMC Infect. Dis. 21:927. doi: 10.1186/s12879-021-06642-2, PMID: - DOI - PMC - PubMed
-
- Amala Reena A. A., Subramaniyan A., Kanungo R. (2017). Biofilm formation as a virulence factor of Acinetobacter baumannii: an emerging pathogen in critical care units. J. Curr. Res. Sci. Med. 3:74. doi: 10.4103/jcrsm.jcrsm_66_17 - DOI
-
- Amin M., Navidifar T., Saleh Shooshtari F., Goodarzi H. (2019). Association of the genes encoding metallo-β-lactamase with the presence of integrons among multidrug-resistant clinical isolates of Acinetobacter baumannii. Infect. Drug Resist. 12, 1171–1180. doi: 10.2147/IDR.S196575, PMID: - DOI - PMC - PubMed
-
- Andrews S. (2010). FastQC: A quality control tool for high throughput sequence data [online]. Available at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
LinkOut - more resources
Full Text Sources
Miscellaneous

