A stay of execution: ATF4 regulation and potential outcomes for the integrated stress response
- PMID: 36825279
- PMCID: PMC9941348
- DOI: 10.3389/fnmol.2023.1112253
A stay of execution: ATF4 regulation and potential outcomes for the integrated stress response
Abstract
ATF4 is a cellular stress induced bZIP transcription factor that is a hallmark effector of the integrated stress response. The integrated stress response is triggered by phosphorylation of the alpha subunit of the eukaryotic initiation factor 2 complex that can be carried out by the cellular stress responsive kinases; GCN2, PERK, PKR, and HRI. eIF2α phosphorylation downregulates mRNA translation initiation en masse, however ATF4 translation is upregulated. The integrated stress response can output two contradicting outcomes in cells; pro-survival or apoptosis. The mechanism for choice between these outcomes is unknown, however combinations of ATF4 heterodimerisation partners and post-translational modifications have been linked to this regulation. This semi-systematic review article covers ATF4 target genes, heterodimerisation partners and post-translational modifications. Together, this review aims to be a useful resource to elucidate the mechanisms controlling the effects of the integrated stress response. Additional putative roles of the ATF4 protein in cell division and synaptic plasticity are outlined.
Keywords: ATF4; ISR; PTM; apoptosis; cell division; dimerization; synaptic plasticity; target genes.
Copyright © 2023 Neill and Masson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

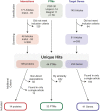
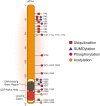
References
-
- Bagheri-Yarmand R., Sinha K. M., Gururaj A. E., Ahmed Z., Rizvi Y. Q., Huang S. C., et al. . (2015). A novel dual kinase function of the RET proto-oncogene negatively regulates activating transcription factor 4-mediated apoptosis. J. Biol. Chem. 290, 11749–11761. doi: 10.1074/jbc.M114.619833 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

