Pathogenesis of Alzheimer's disease: Involvement of the choroid plexus
- PMID: 36825691
- PMCID: PMC10634590
- DOI: 10.1002/alz.12970
Pathogenesis of Alzheimer's disease: Involvement of the choroid plexus
Abstract
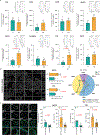
The choroid plexus (ChP) produces and is bathed in the cerebrospinal fluid (CSF), which in aging and Alzheimer's disease (AD) shows extensive proteomic alterations including evidence of inflammation. Considering inflammation hampers functions of the involved tissues, the CSF abnormalities reported in these conditions are suggestive of ChP injury. Indeed, several studies document ChP damage in aging and AD, which nevertheless remains to be systematically characterized. We here report that the changes elicited in the CSF by AD are consistent with a perturbed aging process and accompanied by aberrant accumulation of inflammatory signals and metabolically active proteins in the ChP. Magnetic resonance imaging (MRI) imaging shows that these molecular aberrancies correspond to significant remodeling of ChP in AD, which correlates with aging and cognitive decline. Collectively, our preliminary post-mortem and in vivo findings reveal a repertoire of ChP pathologies indicative of its dysfunction and involvement in the pathogenesis of AD. HIGHLIGHTS: Cerebrospinal fluid changes associated with aging are perturbed in Alzheimer's disease Paradoxically, in Alzheimer's disease, the choroid plexus exhibits increased cytokine levels without evidence of inflammatory activation or infiltrates In Alzheimer's disease, increased choroid plexus volumes correlate with age and cognitive performance.
Keywords: Alzheimer's disease; aging; cerebrospinal fluid; choroid plexus; pathology.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Conflicts of interest
No competing interests.
Figures


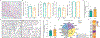

Comment in
-
The partial volume effect of choroid plexus in pathogenesis of Alzheimer's disease.Alzheimers Dement. 2023 Oct;19(10):4756-4757. doi: 10.1002/alz.13123. Epub 2023 May 26. Alzheimers Dement. 2023. PMID: 37235538 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

