Therapeutic Effects of Aloe saponaria against Ulcerative Colitis Induced by Dextran Sulfate Sodium
- PMID: 36826041
- PMCID: PMC9955819
- DOI: 10.3390/cimb45020096
Therapeutic Effects of Aloe saponaria against Ulcerative Colitis Induced by Dextran Sulfate Sodium
Abstract
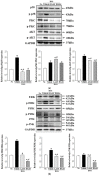
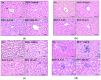
Aloe vera (A. vera) has been studied as a treatment option for ulcerative colitis (UC), but there is a lack of scientific evidence showing whether treatment with Aloe saponaria (A. saponaria) can also be beneficial. To investigate the therapeutic potential of A. saponaria as a treatment for UC, clinical symptoms, histopathological characteristics of the colon, inflammatory response, and toxicity were analyzed in dextran sulfate sodium (DSS)-induced UC mice after administration of aqueous extracts of A. saponaria (AAS) for 7 days. The total polyphenol and tannin content of AAS was 272 µg/g and 163 µg/g, respectively. AAS exhibited significant antioxidant activity. Several clinical symptoms, including body weight, colon length, and hematochezia, remarkably improved in the DSS+AAS treated group compared to the DSS+Vehicle-treated group. In addition, similar improvements were detected in the histopathological characteristics and mucin-secreting ability in the colon of DSS-induced UC mice after the administration of AAS. The levels of infiltrated inflammatory cells and cytokine expression were significantly decreased in a dose-dependent manner in the colon of the DSS+AAS-treated group. These alterations in inflammatory response were accompanied by a significant recovery of the protein kinase C/extracellular signal-regulated kinase (PKC/ERK) and phosphatidylinositol-3-kinase/serine-threonine protein kinase (PI3K/Akt) signaling pathways. However, the levels of key markers for hepatotoxicity and nephrotoxicity consistently remained between those of the DSS+AAS-treated and the No groups. Therefore, the results of the present study provide novel evidence that AAS may improve the clinical symptoms and attenuate the inflammatory response in DSS-induced UC mice and does not have any significant hepatotoxicity or nephrotoxicity.
Keywords: Aloe saponaria; dextran sulfate sodium; inflammation; toxicity; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. - PubMed
-
- Gajendran M., Loganathan P., Jimenez G., Catinella A.P., Ng N., Umapathy C., Ziade N., Hashash J.G. A comprehensive review and update on ulcerative colitis. Dis. A Mon. 2019;65:100851. - PubMed
-
- Turner D., Levine A., Escher J.C., Griffiths A.M., Russell R.K., Dignass A., Dias J.A., Bronsky J., Braegger C.P., Cucchiara S., et al. Management of pediatric ulcerative colitis: Joint ECCO and ESPGHAN evidence-based consensus guidelines. J. Pediatr. Gastroenterol. Nutr. 2012;55:340–361. doi: 10.1097/MPG.0b013e3182662233. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

