Neuroprotective Effects of Geopung-Chunghyuldan Based on Its Salvianolic Acid B Content Using an In Vivo Stroke Model
- PMID: 36826049
- PMCID: PMC9955915
- DOI: 10.3390/cimb45020104
Neuroprotective Effects of Geopung-Chunghyuldan Based on Its Salvianolic Acid B Content Using an In Vivo Stroke Model
Abstract
Background: Geopung-Chunghyuldan (GCD) has neuroprotective properties. Salviae miltiorrhizae Radix plays an essential role in GCD's effect. The Salviae miltiorrhizae Radix marker compound is salvianolic acid B; however, its content is not uniform among samples. This study aimed to evaluate the neuroprotective effects of GCD based on salvianolic acid B content.
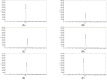
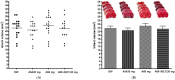
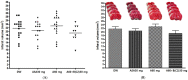
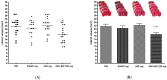
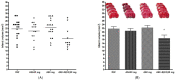
Methods: The neuroprotective effects of GCD based on the salvianolic acid B content were evaluated by measuring infarct volume 24 h after permanent middle cerebral artery occlusion in an in vivo stroke model. For the experimental group, each GCD was administered immediately before surgery. The control groups were administered distilled water and aspirin (30 mg/kg) in the same way. The salvianolic acid B content in five types of Salviae Miltiorrhizae Radix (two Chinese and three Korean regions) based on different cultivation regions was analyzed by high-performance liquid chromatography.
Results: Three samples met the Korean and Chinese Pharmacopeia standards for salvianolic acid B. However, two samples did not. GCDs with high salvianolic acid B showed marked neuroprotective effects compared to the control groups, whereas GCDs with low salvianolic acid B did not.
Conclusions: The salvianolic acid B content of Salviae miltiorrhizae Radix affects the neuroprotection effect of GCD. Stable, raw Salviae miltiorrhizae Radix is essential for GCD homogenization.
Keywords: Geopung-Chunghyuldan; Salviae miltiorrhizae Radix; neuroprotection; permanent middle cerebral artery occlusion; salvianolic acid B.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

