Management of Marginal Zone Lymphoma: A Canadian Perspective
- PMID: 36826096
- PMCID: PMC9955247
- DOI: 10.3390/curroncol30020135
Management of Marginal Zone Lymphoma: A Canadian Perspective
Abstract
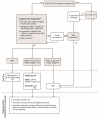
Marginal zone lymphomas (MZL) are a rare, heterogenous group of lymphomas, accounting for 5-17% of indolent non-Hodgkin lymphomas in the western world. They can be further divided into three subtypes: extranodal MZL, splenic MZL, and nodal MZL. These subtypes differ in clinical presentation and behavior, which influences how they are managed. There is currently no standard of care for the treatment of MZL, owing to the difficulty in conducting phase 3 randomized trials in MZL, and the fact that there are limited data on the efficacy of therapy in individual subtypes. Treatment practices are thus largely borrowed from other indolent lymphomas and are based on patient and disease characteristics, as well as access to therapy. This review summarizes the Canadian treatment landscape for MZL and how these therapies may be sequenced in practice.
Keywords: Bruton’s tyrosine kinase inhibitor; anti-CD20 monoclonal antibody; chemoimmunotherapy; marginal zone lymphoma; mucosa-associated lymphoid tissue lymphoma.
Conflict of interest statement
A.P. (Anthea Peters) has served on advisory boards for Roche, Janssen, BeiGene, AstraZeneca, AbbVie, and Incyte. M-M.K. has served on advisory boards for Bristol Myers Squibb, Taiho, Seattle Genetics, BeiGene, Astra Zeneca. A.K. has served on advisory boards for BeiGene and Janssen. S.D. has received funding from BeiGene Inc. for medical writing services. The funders had no role in the content development or writing of the manuscript. A.P(r). has received honoraria from AstraZeneca and AbbVie.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

