In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis
- PMID: 36826282
- PMCID: PMC9957534
- DOI: 10.3390/gels9020112
In Vitro and In Vivo Investigation of a Dual-Targeted Nanoemulsion Gel for the Amelioration of Psoriasis
Abstract
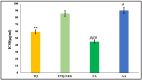
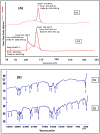
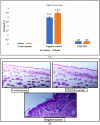
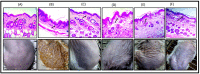
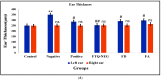
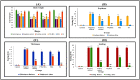
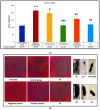
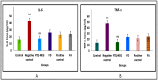
Psoriasis, due to its unique pathological manifestations and the limited success of existing therapeutic modalities, demands dedicated domain research. Our group has developed nanotherapeutics consisting of bioactives such as Thymoquinone (TQ) and Fulvic acid (FA), which have been successfully incorporated into a Nanoemulsion gel (NEG), taking kalonji oil as oil phase. The composition is aimed at ameliorating psoriasis with better therapeutic outcomes. TQ is a natural bio-active that has been linked to anti-psoriatic actions. FA has anti-inflammatory actions due to its free radical and oxidant-scavenging activity. Our previous publication reports the formulation development of the NEG, where we overcame the pharmaco-technical limitations of combining the above two natural bioactives. In vitro evaluation of the optimized NEG was carried out, which showed an enhanced dissolution rate and skin permeation of TQ. This work furthers the pharmaceutical progression of dual-targeted synergistic NEG to treat psoriasis. A suitable animal model, BALB/c mice, has been used to conduct the in vivo studies, which revealed the effective anti-psoriatic action of TQ. Molecular docking studies corroborated the results and revealed a good binding affinity for both the targets of TNF-α (Tumor necrosis factor) and IL-6 (Interlukin-6). Tissue uptake by Confocal laser scanning microscopy (CLSM), a skin interaction study of the gel formulation, and an antioxidant free radical scavenging assay (1-1 Diphenyl-2-picrylhydrazyl DPPH) were also carried out. It was concluded that the NEG may be effective in treating psoriasis with minimal side effects.
Keywords: antioxidant; imiquimod-induced psoriatic mice model; immunohistochemistry; nanoemulsion gel; skin histopathology; thymoquinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

