Development of Adapalene Loaded Liposome Based Gel for Acne
- PMID: 36826305
- PMCID: PMC9956198
- DOI: 10.3390/gels9020135
Development of Adapalene Loaded Liposome Based Gel for Acne
Abstract
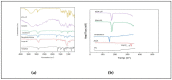
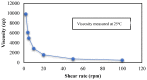
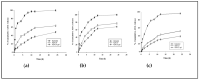
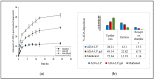
Retinoids are considered the mainstay treatment for moderate to severe acne. Adapalene, a third-generation retinoid, has physiochemical properties which hinder the effective delivery of the drug to the skin. Therefore, the current study aimed to develop and evaluate adapalene liposomal loaded gel (ADA-LP gel) for the effective management of acne to improve tolerability and delivery to targeted sites as compared to the conventional dosage form of the drug. A novel spontaneous phase transition method (SPT) was used to formulate liposomes. Liposomal formulation (ADA-LP) was prepared and optimized based on particle size, zeta potential, and PDI. Optimized formulation was further characterized by different techniques and loaded into Carbopol gel. In vitro drug release, ex vivo permeation, and in vivo studies were performed using the prepared adapalene-loaded liposomal-based gel. The in vivo study was done employing the testosterone-induced acne model in mice. The optimized formulation had a size of 181 nm, PDI 0.145, and a zeta potential of -35 mV, indicating that the formulation was stable. Encapsulation efficiency was 89.69 ± 0.5%. ADA-LPs were loaded into the gel. Prepared ADA-LP showed a 79 ± 0.02% release of drug in a sustained manner, within 24 h. The ex vivo permeability study showed a total of 43 ± 0.06 µg/cm2 of drug able to permeate through the skin within 24 h. Moreover, only 28.27 ± 0.04% was retained on the epidermis. The developed ADA-LP gel showed significant improvement in the acne lesions in mice with no visible scars and inflammation on the skin. Therefore, ADA-LP-based gel could be a promising carrier system for the safe and effective delivery of Adapalene.
Keywords: Carbopol; adapalene; animal model; liposomes; optimization; particle size.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
-
- Dreno B., Amici J.M., Demessant-Flavigny A.L., Wright C., Taieb C., Desai S.R., Alexis A. The impact of acne, atopic dermatitis, skin toxicities and scars on quality of life and the importance of a holistic treatment approach. Clin. Cosmet. Investig. Dermatol. 2021;14:623. doi: 10.2147/CCID.S315846. - DOI - PMC - PubMed
-
- Afzal M., Alharbi K.S., Alruwaili N.K., Al-Abassi F.A., Al-Malki A.A.L., Kazmi I., Kumar V., Kamal M.A., Nadeem M.S., Aslam M. Seminars in Cancer Biology. Academic Press; Cambridge, MA, USA: 2021. Nanomedicine in treatment of breast cancer—A challenge to conventional therapy; pp. 279–292. - PubMed
-
- Khattak M.I.K., Ahmed N., Umer M.F., Riaz A., Ahmad N.M., Khan G.M. Chloroform-Injection (CI) and Spontaneous-Phase-Transition (SPT) Are Novel Methods, Simplifying the Fabrication of Liposomes with Versatile Solution to Cholesterol Content and Size Distribution. Pharmaceutics. 2020;12:1065. doi: 10.3390/pharmaceutics12111065. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

