Nanocomposite Hydrogels as Functional Extracellular Matrices
- PMID: 36826323
- PMCID: PMC9957407
- DOI: 10.3390/gels9020153
Nanocomposite Hydrogels as Functional Extracellular Matrices
Abstract
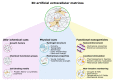
Over recent years, nano-engineered materials have become an important component of artificial extracellular matrices. On one hand, these materials enable static enhancement of the bulk properties of cell scaffolds, for instance, they can alter mechanical properties or electrical conductivity, in order to better mimic the in vivo cell environment. Yet, many nanomaterials also exhibit dynamic, remotely tunable optical, electrical, magnetic, or acoustic properties, and therefore, can be used to non-invasively deliver localized, dynamic stimuli to cells cultured in artificial ECMs in three dimensions. Vice versa, the same, functional nanomaterials, can also report changing environmental conditions-whether or not, as a result of a dynamically applied stimulus-and as such provide means for wireless, long-term monitoring of the cell status inside the culture. In this review article, we present an overview of the technological advances regarding the incorporation of functional nanomaterials in artificial extracellular matrices, highlighting both passive and dynamically tunable nano-engineered components.
Keywords: biosensing; nanocomposite hydrogel; nanoparticles; remote stimulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Reversible dynamic mechanics of hydrogels for regulation of cellular behavior.Acta Biomater. 2021 Dec;136:88-98. doi: 10.1016/j.actbio.2021.09.032. Epub 2021 Sep 23. Acta Biomater. 2021. PMID: 34563721 Free PMC article.
-
Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering.Int J Biol Macromol. 2018 Mar;108:383-390. doi: 10.1016/j.ijbiomac.2017.12.032. Epub 2017 Dec 7. Int J Biol Macromol. 2018. PMID: 29225174
-
Engineering Cellular Microenvironments with Photo- and Enzymatically Responsive Hydrogels: Toward Biomimetic 3D Cell Culture Models.Acc Chem Res. 2017 Apr 18;50(4):703-713. doi: 10.1021/acs.accounts.6b00543. Epub 2017 Mar 27. Acc Chem Res. 2017. PMID: 28345876
-
Exploiting the role of nanoparticles for use in hydrogel-based bioprinting applications: concept, design, and recent advances.Biomater Sci. 2021 Sep 28;9(19):6337-6354. doi: 10.1039/d1bm00605c. Biomater Sci. 2021. PMID: 34397056 Review.
-
Engineering Stem Cell-Derived Extracellular Matrices: Decellularization, Characterization, and Biological Function.Tissue Eng Part B Rev. 2020 Oct;26(5):402-422. doi: 10.1089/ten.TEB.2019.0349. Epub 2020 Apr 28. Tissue Eng Part B Rev. 2020. PMID: 32220216 Review.
Cited by
-
Hydrogel-based biomaterials for brain regeneration after stroke: Gap to clinical translation.Biomater Transl. 2025 Jun 25;6(2):165-180. doi: 10.12336/bmt.24.00020. eCollection 2025. Biomater Transl. 2025. PMID: 40641992 Free PMC article. Review.
-
Cellulose-Based Hybrid Hydrogels for Tissue Engineering Applications: A Sustainable Approach.Gels. 2025 Jun 6;11(6):438. doi: 10.3390/gels11060438. Gels. 2025. PMID: 40558737 Free PMC article. Review.
-
Hydrogel Contact Lenses Embedded with Amine-Functionalized Large-Pore Mesoporous Silica Nanoparticles with Extended Hyaluronic Acid Release.Nanomaterials (Basel). 2023 Aug 28;13(17):2441. doi: 10.3390/nano13172441. Nanomaterials (Basel). 2023. PMID: 37686949 Free PMC article.
-
Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy.Gels. 2023 Jun 30;9(7):533. doi: 10.3390/gels9070533. Gels. 2023. PMID: 37504412 Free PMC article. Review.
-
Stimuli-Responsive Nanocomposite Hydrogels for Oral Diseases.Gels. 2024 Jul 18;10(7):478. doi: 10.3390/gels10070478. Gels. 2024. PMID: 39057501 Free PMC article. Review.
References
-
- Parks W.C., Mecham R.P., editors. Extracellular Matrix Degradation. Volume 1. Springer; Berlin/Heidelberg, Germany: 2011.
-
- Cai S., Wu C., Yang W., Liang W., Yu H., Liu L. Recent Advance in Surface Modification for Regulating Cell Adhesion and Behaviors. Nanotechnol. Rev. 2020;9:971–989. doi: 10.1515/ntrev-2020-0076. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

