Antibacterial Activity and Cell Viability of Biomimetic Magnesian Calcite Coatings on Biodegradable Mg
- PMID: 36826897
- PMCID: PMC9963250
- DOI: 10.3390/jfb14020098
Antibacterial Activity and Cell Viability of Biomimetic Magnesian Calcite Coatings on Biodegradable Mg
Abstract
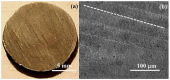
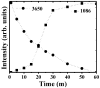
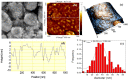
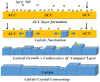
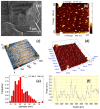
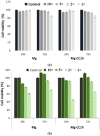
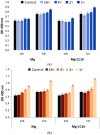
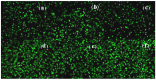
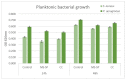
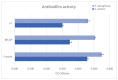
Mg is a material of choice for biodegradable implants. The main challenge for using Mg in temporary implants is to provide protective surfaces that mitigate its rapid degradation in biological fluids and also confer sufficient cytocompatibility and bacterial resistance to Mg-coated surfaces. Even though carbonate mineralization is the most important source of biominerals, such as the skeletons and shells of many marine organisms, there has been little success in the controlled growth of carbonate layers by synthetic processes. We present here the formation mechanism, antibacterial activity, and cell viability of magnesian calcite biomimetic coatings grown on biodegradable Mg via a green, one-step route. Cell compatibility assessment showed cell viability higher than 80% after 72 h using fibroblast cells (NCTC, clone L929) and higher than 60% after 72 h using human osteoblast-like cells (SaOS-2); the cells displayed a normal appearance and a density similar to the control sample. Antimicrobial potential evaluation against both Gram-positive (Staphylococcus aureus (ATCC 25923)) and Gram-negative (Pseudomonas aeruginosa (ATCC 27853)) strains demonstrated that the coated samples significantly inhibited bacterial adhesion and biofilm formation compared to the untreated control. Calcite coatings grown on biodegradable Mg by a single coating process showed the necessary properties of cell compatibility and bacterial resistance for application in surface-modified Mg biomaterials for temporary implants.
Keywords: CaCO3; amorphous calcium carbonate (ACC); antibacterial; bone scaffolds; cell viability; corrosion protective film; resorbable biomaterial.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
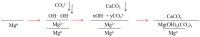





References
-
- Song G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007;49:1696–1701. doi: 10.1016/j.corsci.2007.01.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

