Effectiveness of BMP-2 and PDGF-BB Adsorption onto a Collagen/Collagen-Magnesium-Hydroxyapatite Scaffold in Weight-Bearing and Non-Weight-Bearing Osteochondral Defect Bone Repair: In Vitro, Ex Vivo and In Vivo Evaluation
- PMID: 36826910
- PMCID: PMC9961206
- DOI: 10.3390/jfb14020111
Effectiveness of BMP-2 and PDGF-BB Adsorption onto a Collagen/Collagen-Magnesium-Hydroxyapatite Scaffold in Weight-Bearing and Non-Weight-Bearing Osteochondral Defect Bone Repair: In Vitro, Ex Vivo and In Vivo Evaluation
Abstract
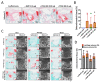
Despite promising clinical results in osteochondral defect repair, a recently developed bi-layered collagen/collagen-magnesium-hydroxyapatite scaffold has demonstrated less optimal subchondral bone repair. This study aimed to improve the bone repair potential of this scaffold by adsorbing bone morphogenetic protein 2 (BMP-2) and/or platelet-derived growth factor-BB (PDGF-BB) onto said scaffold. The in vitro release kinetics of BMP-2/PDGF-BB demonstrated that PDGF-BB was burst released from the collagen-only layer, whereas BMP-2 was largely retained in both layers. Cell ingrowth was enhanced by BMP-2/PDFG-BB in a bovine osteochondral defect ex vivo model. In an in vivo semi-orthotopic athymic mouse model, adding BMP-2 or PDGF-BB increased tissue repair after four weeks. After eight weeks, most defects were filled with bone tissue. To further investigate the promising effect of BMP-2, a caprine bilateral stifle osteochondral defect model was used where defects were created in weight-bearing femoral condyle and non-weight-bearing trochlear groove locations. After six months, the adsorption of BMP-2 resulted in significantly less bone repair compared with scaffold-only in the femoral condyle defects and a trend to more bone repair in the trochlear groove. Overall, the adsorption of BMP-2 onto a Col/Col-Mg-HAp scaffold reduced bone formation in weight-bearing osteochondral defects, but not in non-weight-bearing osteochondral defects.
Keywords: animal model; biocompatible materials; bone morphogenetic proteins; osteochondral lesion; platelet-derived growth factor; regenerative medicine; tissue engineering; weight-bearing.
Conflict of interest statement
M. Maraglino Misciagna, L. Dolcini, L. Forte, and C. De Luca work at Fin-Ceramica Faenza S.p.A, a company that develops, manufactures, and markets collagen/collagen-magnesium-hydroxyapatite scaffolds for orthopaedic and spinal applications. The other authors declare no conflicts of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

