A Real-Time Polyp-Detection System with Clinical Application in Colonoscopy Using Deep Convolutional Neural Networks
- PMID: 36826945
- PMCID: PMC9967208
- DOI: 10.3390/jimaging9020026
A Real-Time Polyp-Detection System with Clinical Application in Colonoscopy Using Deep Convolutional Neural Networks
Abstract
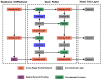
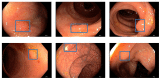
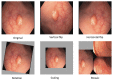
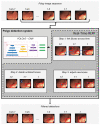
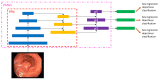
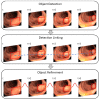
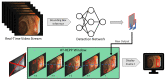
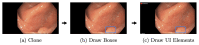
Colorectal cancer (CRC) is a leading cause of cancer-related deaths worldwide. The best method to prevent CRC is with a colonoscopy. During this procedure, the gastroenterologist searches for polyps. However, there is a potential risk of polyps being missed by the gastroenterologist. Automated detection of polyps helps to assist the gastroenterologist during a colonoscopy. There are already publications examining the problem of polyp detection in the literature. Nevertheless, most of these systems are only used in the research context and are not implemented for clinical application. Therefore, we introduce the first fully open-source automated polyp-detection system scoring best on current benchmark data and implementing it ready for clinical application. To create the polyp-detection system (ENDOMIND-Advanced), we combined our own collected data from different hospitals and practices in Germany with open-source datasets to create a dataset with over 500,000 annotated images. ENDOMIND-Advanced leverages a post-processing technique based on video detection to work in real-time with a stream of images. It is integrated into a prototype ready for application in clinical interventions. We achieve better performance compared to the best system in the literature and score a F1-score of 90.24% on the open-source CVC-VideoClinicDB benchmark.
Keywords: automation; deep learning; endoscopy; gastroenterology; machine learning; object detection; real-time; video object detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



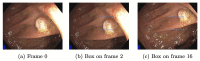



References
-
- Heresbach D., Barrioz T., Lapalus M., Coumaros D., Bauret P., Potier P., Sautereau D., Boustière C., Grimaud J., Barthélémy C., et al. Miss rate for colorectal neoplastic polyps: A prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–290. doi: 10.1055/s-2007-995618. - DOI - PubMed
LinkOut - more resources
Full Text Sources

