Stellettin B Induces Cell Death in Bladder Cancer Via Activating the Autophagy/DAPK2/Apoptosis Signaling Cascade
- PMID: 36827114
- PMCID: PMC9966069
- DOI: 10.3390/md21020073
Stellettin B Induces Cell Death in Bladder Cancer Via Activating the Autophagy/DAPK2/Apoptosis Signaling Cascade
Abstract
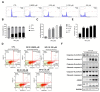
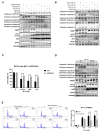
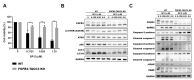
Bladder cancer (BC) is one of the most prevalent cancers worldwide. However, the recurrence rate and five-year survival rate have not been significantly improved in advanced BC, and new therapeutic strategies are urgently needed. The anticancer activity of stellettin B (SP-2), a triterpene isolated from the marine sponge Rhabdastrella sp., was evaluated with the MTT assay as well as PI and Annexin V/7-AAD staining. Detailed mechanisms were elucidated through an NGS analysis, protein arrays, and Western blotting. SP-2 suppressed the viability of BC cells without severe toxicity towards normal uroepithelial cells, and it increased apoptosis with the activation of caspase 3/8/9, PARP, and γH2AX. The phosphorylation of FGFR3 and its downstream targets were downregulated by SP-2. Meanwhile, it induced autophagy in BC cells as evidenced by LC3-II formation and p62 downregulation. The inhibition of autophagy using pharmacological inhibitors or through an ATG5-knockout protected RT-112 cells from SP-2-induced cell viability suppression and apoptosis. In addition, the upregulation of DAPK2 mRNA and protein expression also contributed to SP-2-induced cytotoxicity and apoptosis. In RT-112 cells, an FGFR3-TACC3-knockout caused the downregulation of DAPK2, autophagy, and apoptosis. In conclusion, this is the first study demonstrating that SP-2 exhibits potent anti-BC activity by suppressing the FGFR3-TACC3/Akt/mTOR pathway, which further activates a novel autophagy/DAPK2/apoptosis signaling cascade.
Keywords: DAPK2; apoptosis; autophagy; bladder cancer; stellettin B.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
In vitro antitumor activity of stellettin B, a triterpene from marine sponge Jaspis stellifera, on human glioblastoma cancer SF295 cells.Mar Drugs. 2014 Jul 15;12(7):4200-13. doi: 10.3390/md12074200. Mar Drugs. 2014. PMID: 25028795 Free PMC article.
-
Stellettin B-Induced Oral Cancer Cell Death via Endoplasmic Reticulum Stress-Mitochondrial Apoptotic and Autophagic Signaling Pathway.Int J Mol Sci. 2022 Aug 8;23(15):8813. doi: 10.3390/ijms23158813. Int J Mol Sci. 2022. PMID: 35955957 Free PMC article.
-
Autophagy induction enhances tetrandrine-induced apoptosis via the AMPK/mTOR pathway in human bladder cancer cells.Oncol Rep. 2017 Nov;38(5):3137-3143. doi: 10.3892/or.2017.5988. Epub 2017 Sep 21. Oncol Rep. 2017. PMID: 29048631
-
PDB-1 from Potentilla discolor Bunge induces apoptosis and autophagy by downregulating the PI3K/Akt/mTOR signaling pathway in A549 cells.Biomed Pharmacother. 2020 Sep;129:110378. doi: 10.1016/j.biopha.2020.110378. Epub 2020 Jun 13. Biomed Pharmacother. 2020. PMID: 32544818
-
The role of DAPK2 as a key regulatory element in various human cancers: a systematic review.Mol Biol Rep. 2024 Aug 6;51(1):886. doi: 10.1007/s11033-024-09761-6. Mol Biol Rep. 2024. PMID: 39105958
Cited by
-
TRAIL-mediated signaling in bladder cancer: realization of clinical efficacy of TRAIL-based therapeutics in medical oncology.Med Oncol. 2023 Jul 11;40(8):236. doi: 10.1007/s12032-023-02078-7. Med Oncol. 2023. PMID: 37432489 Review.
-
Exploring the Targets and Molecular Mechanisms of Curcumin for the Treatment of Bladder Cancer Based on Network Pharmacology, Molecular Docking and Molecular Dynamics.Mol Biotechnol. 2025 May;67(5):2138-2159. doi: 10.1007/s12033-024-01190-x. Epub 2024 Jun 1. Mol Biotechnol. 2025. PMID: 38822913
-
FGFR inhibitors promote the autophagic degradation of IFN-γ-induced PD-L1 and alleviate the PD-L1-mediated transcriptional suppression of FGFR3-TACC3 in non-muscle-invasive bladder cancer.Cell Death Dis. 2025 Jul 2;16(1):485. doi: 10.1038/s41419-025-07821-8. Cell Death Dis. 2025. PMID: 40603902 Free PMC article.
-
Hinokiflavone is a novel CK2 inhibitor promoting apoptosis and synergizing with chemotherapeutic agents in cisplatin resistant bladder cancer cells.Sci Rep. 2025 Jul 1;15(1):20922. doi: 10.1038/s41598-025-06543-3. Sci Rep. 2025. PMID: 40594996 Free PMC article.
-
Marine-Derived Leads as Anticancer Candidates by Disrupting Hypoxic Signaling through Hypoxia-Inducible Factors Inhibition.Mar Drugs. 2024 Mar 23;22(4):143. doi: 10.3390/md22040143. Mar Drugs. 2024. PMID: 38667760 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

