Enzyme Inhibitors from Gorgonians and Soft Corals
- PMID: 36827145
- PMCID: PMC9963996
- DOI: 10.3390/md21020104
Enzyme Inhibitors from Gorgonians and Soft Corals
Abstract
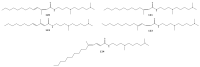
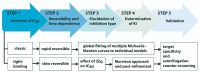
For decades, gorgonians and soft corals have been considered promising sources of bioactive compounds, attracting the interest of scientists from different fields. As the most abundant bioactive compounds within these organisms, terpenoids, steroids, and alkaloids have received the highest coverage in the scientific literature. However, enzyme inhibitors, a functional class of bioactive compounds with high potential for industry and biomedicine, have received much less notoriety. Thus, we revised scientific literature (1974-2022) on the field of marine natural products searching for enzyme inhibitors isolated from these taxonomic groups. In this review, we present representative enzyme inhibitors from an enzymological perspective, highlighting, when available, data on specific targets, structures, potencies, mechanisms of inhibition, and physiological roles for these molecules. As most of the characterization studies for the new inhibitors remain incomplete, we also included a methodological section presenting a general strategy to face this goal by accomplishing STRENDA (Standards for Reporting Enzymology Data) project guidelines.
Keywords: STRENDA guidelines; enzyme inhibitors; enzyme kinetics; gorgonian; inhibitor characterization; natural products; soft coral.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
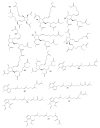
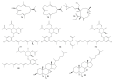
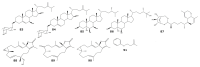
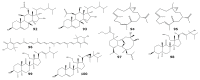

Similar articles
-
Terpenoids from Marine Soft Coral of the Genus Xenia in 1977 to 2019.Molecules. 2020 Nov 18;25(22):5386. doi: 10.3390/molecules25225386. Molecules. 2020. PMID: 33217924 Free PMC article. Review.
-
Recent Updates on Sinularia Soft Coral.Mini Rev Med Chem. 2022;22(8):1152-1196. doi: 10.2174/1389557521666210927152249. Mini Rev Med Chem. 2022. PMID: 34579632
-
Coral and Coral-Associated Microorganisms: A Prolific Source of Potential Bioactive Natural Products.Mar Drugs. 2019 Aug 11;17(8):468. doi: 10.3390/md17080468. Mar Drugs. 2019. PMID: 31405226 Free PMC article. Review.
-
Recent Updates on Corals from Nephtheidae.Chem Biodivers. 2019 Jun;16(6):e1800692. doi: 10.1002/cbdv.201800692. Epub 2019 May 20. Chem Biodivers. 2019. PMID: 30957385 Review.
-
Diterpenes from gorgonian corals.Nat Prod Rep. 2009 May;26(5):681-710. doi: 10.1039/b821918b. Epub 2009 Mar 25. Nat Prod Rep. 2009. PMID: 19387501 Review.
Cited by
-
Theoretical study on the structures and pharmacokinetic evaluation of verticillane-type diterpenes from soft coral Heteroxenia ghardaqensis.BMC Chem. 2025 May 11;19(1):122. doi: 10.1186/s13065-025-01499-x. BMC Chem. 2025. PMID: 40350455 Free PMC article.
-
Anthozoan Chemical Defenses: Integrating Compounds, Enzymatic Activities, and Omics-Based Discoveries.Int J Mol Sci. 2025 Jun 25;26(13):6109. doi: 10.3390/ijms26136109. Int J Mol Sci. 2025. PMID: 40649883 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

