Modified Akuamma Alkaloids with Increased Potency at the Mu-opioid Receptor
- PMID: 36827198
- PMCID: PMC10037270
- DOI: 10.1021/acs.jmedchem.2c01707
Modified Akuamma Alkaloids with Increased Potency at the Mu-opioid Receptor
Abstract
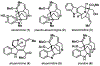
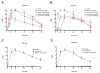
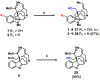
Akuammine (1) and pseudoakuammigine (2) are indole alkaloids found in the seeds of the akuamma tree (Picralima nitida). Both alkaloids are weak agonists of the mu opioid receptor (μOR); however, they produce minimal effects in animal models of antinociception. To probe the interactions of 1 and 2 at the opioid receptors, we have prepared a collection of 22 semisynthetic derivatives. Evaluation of this collection at the μOR and kappa opioid receptor (κOR) revealed structural-activity relationship trends and derivatives with improved potency at the μOR. Most notably, the introduction of a phenethyl moiety to the N1 of 2 produces a 70-fold increase in potency and a 7-fold increase in selectivity for the μOR. The in vitro potency of this compound resulted in increased efficacy in the tail-flick and hot-plate assays of antinociception. The improved potency of these derivatives highlights the promise of exploring natural product scaffolds to probe the opioid receptors.
Figures

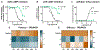

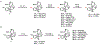

References
-
- Volkow ND; Collins FS The Role of Science in Addressing the Opioid Crisis. N. Engl. J. Med 2017, 377, 391–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials

