A microbiota and dietary metabolite integrates DNA repair and cell death to regulate embryo viability and aneuploidy during aging
- PMID: 36827370
- PMCID: PMC9956122
- DOI: 10.1126/sciadv.ade8653
A microbiota and dietary metabolite integrates DNA repair and cell death to regulate embryo viability and aneuploidy during aging
Abstract
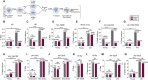
During aging, environmental stressors and mutations along with reduced DNA repair cause germ cell aneuploidy and genome instability, which limits fertility and embryo development. Benevolent commensal microbiota and dietary plants secrete indoles, which improve healthspan and reproductive success, suggesting regulation of germ cell quality. We show that indoles prevent aneuploidy and promote DNA repair and embryo viability, which depends on age and genotoxic stress levels and affects embryo quality across generations. In young animals or with low doses of radiation, indoles promote DNA repair and embryo viability; however, in older animals or with high doses of radiation, indoles promote death of the embryo. These studies reveal a previously unknown quality control mechanism by which indole integrates DNA repair and cell death responses to preclude germ cell aneuploidy and ensure transgenerational genome integrity. Such regulation affects healthy aging, reproductive senescence, cancer, and the evolution of genetic diversity in invertebrates and vertebrates.
Figures






References
-
- T. E. Schmid, B. Eskenazi, A. Baumgartner, F. Marchetti, S. Young, R. Weldon, D. Anderson, A. J. Wyrobek, The effects of male age on sperm DNA damage in healthy non-smokers. Hum. Reprod. 22, 180–187 (2007). - PubMed
-
- P. Nurse, Y. Masui, L. Hartwell, Understanding the cell cycle. Nat. Med. 4, 1103–1106 (1998). - PubMed
-
- L. Stergiou, M. O. Hengartner, Death and more: DNA damage response pathways in the nematode C. elegans. Cell Death Differ. 11, 21–28 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

