Targeted Microglial Attenuation through Dendrimer-Drug Conjugates Improves Glaucoma Neuroprotection
- PMID: 36827603
- PMCID: PMC10189638
- DOI: 10.1021/acs.biomac.2c01381
Targeted Microglial Attenuation through Dendrimer-Drug Conjugates Improves Glaucoma Neuroprotection
Abstract
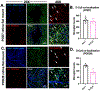
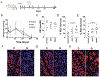
Retinal microglial/macrophage activation and optic nerve (ON) microglial/macrophage activation are glaucoma biomarkers and potential therapeutic targets for this blinding disease. We report targeting of activated microglia by PAMAM dendrimers in a rat glaucoma model and neuroprotection by N-acetylcysteine-conjugated dendrimer (D-NAC) conjugates in a post-injury rescue experiment. Intravitreally delivered fluorescently labeled dendrimer (D-Cy5) conjugates targeted and were retained in Iba-1-positive cells (90% at 7 days and 55% after 28 days) in the retina following intraocular pressure (IOP) elevation, while systemically delivered D-Cy5 targeted ON cells. A single intravitreal D-NAC dose given 1 week after IOP elevation significantly reduced transcription of pro-inflammatory (IL-6, MCP-1, IL-1β) and A1 astrocyte (Serping1, Fkbp5, Amigo2) markers and increased survival of retinal ganglion cells (39 ± 12%) versus BSS- (20 ± 15%, p = 0.02) and free NAC-treated (26 ± 14%, p = 0.15) eyes. These results highlight the potential of dendrimer-targeted microglia and macrophages for early glaucoma detection and as a neuroprotective therapeutic target.
Conflict of interest statement
The authors declare the following competing financial interest(s): Under a license agreement between Ashvattha Therapeutics, Inc and the Johns Hopkins University, Dr. Kannan Rangaramanujam and the University are entitled to royalty distributions related to dendrimer platform technology described in the study. Dr. Rangaramanujam and spouse Dr. S. Kannan are founders of and holds equity in Ashvattha Therapeutics, Inc. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies.
Figures





References
-
- Tham YC; Li X; Wong TY; Quigley HA; Aung T; Cheng CY Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. - PubMed
-
- Kass MA; Heuer DK; Higginbotham EJ; Johnson CA; Keltner JL; Miller JP; Parrish RK II; Wilson MR; Gordon MO The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol 2002, 120, 701–713. - PubMed
-
- Leske MC; Hyman L; Hussein M; Heijl A; Bengtsson B Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol 1999, 127, 625–626. - PubMed
-
- Leske MC; Heijl A; Hussein M; Bengtsson B; Hyman L; Komaroff E; Group EMGT. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch. Ophthalmol 2003, 121, 48–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

