Impact of second primary malignancy post-autologous transplantation on outcomes of multiple myeloma: a CIBMTR analysis
- PMID: 36827681
- PMCID: PMC10275699
- DOI: 10.1182/bloodadvances.2022009138
Impact of second primary malignancy post-autologous transplantation on outcomes of multiple myeloma: a CIBMTR analysis
Abstract
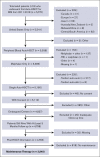
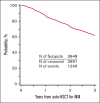
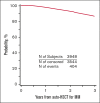
The overall survival (OS) has improved significantly in multiple myeloma (MM) over the last decade with the use of proteasome inhibitor and immunomodulatory drug-based combinations, followed by high-dose melphalan and autologous hematopoietic stem cell transplantation (auto-HSCT) and subsequent maintenance therapies in eligible newly diagnosed patients. However, clinical trials using auto-HSCT followed by lenalidomide maintenance have shown an increased risk of second primary malignancies (SPM), including second hematological malignancies (SHM). We evaluated the impact of SPM and SHM on progression-free survival (PFS) and OS in patients with MM after auto-HSCT using CIBMTR registry data. Adult patients with MM who underwent first auto-HSCT in the United States with melphalan conditioning regimen from 2011 to 2018 and received maintenance therapy were included (n = 3948). At a median follow-up of 37 months, 175 (4%) patients developed SPM, including 112 (64%) solid, 36 (20%) myeloid, 24 (14%) SHM, not otherwise specified, and 3 (2%) lymphoid malignancies. Multivariate analysis demonstrated that SPM and SHM were associated with an inferior PFS (hazard ratio [HR] 2.62, P < .001 and HR 5.01, P < .001, respectively) and OS (HR 3.85, P < .001 and HR 8.13, P < .001, respectively). In patients who developed SPM and SHM, MM remained the most frequent primary cause of death (42% vs 30% and 53% vs 18%, respectively). We conclude the development of SPM and SHM leads to a poor survival in patients with MM and is an important survivorship challenge. Given the median survival for MM continues to improve, continued vigilance is needed to assess the risks of SPM and SHM with maintenance therapy post-auto-HSCT.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: B.K.R. reports advisory board participation for Astellas and Pfizer. M.V.S. reports grants or contracts in the form of institutional research funding from BMS, Astellas, and MRKR Therapeutics. A.D. reports compensation for a clinical trial as site PI for AbbVie, Caelum, TeneoBio, Takeda, Sanofi, Janssen, and Prothena; ad board for BMS; consulting for Prothena; Independent Review Committee participation for Janssen; Payments: A.D. is a member of an independent review committee for a phase 3 clinical trial. In this ongoing role, A.D. performs blinded review of disease response. A.D. is compensated for every hour of consulting work, and this has amounted to more than $5000 compensation in a year. G.K. reports advisory board participation and has received honoraria from BMS, Sanofi, Cellectar and Arcellx. R.B. reports consulting fees for Clearview Healthcare Partners, Guidepoint Global, Genentech/Roche, Janssen Oncology, Sanofi Pasteur, and SparkCures; and payment or honoraria from Clinical Care Options, Curio Science, and i3 Health. A.S. reports royalties or licenses from In8Bio Inc; and consulting fees from Kite Pharma, Magenta Therapeutics, Incyte Pharma, and CareDx. H.M. reports advisory board participation for Janssen, GSK, BMS, Takeda, Pfizer, Amgen, AbbVie, Sanofi, and FORUS; and research funding from Janssen. M.B. reports research support for unrelated project paid to institute from Novartis. H.S.M. reports advisory board participation for CRISPR Therapeutics and Senti Biosciences. C.S. reports consulting fees from Jazz Pharmaceuticals; payment or honoraria from Iowa Oncology Society; and participation on a data safety monitoring board or advisory board for GSK. S.A.H. reports consulting fees from Janssen. D.H.V. reports speakers bureau participation for Amgen, Takeda, BMS, GSK, Karyopharm, Janssen, and Sanofi; participation on a data safety monitoring board or advisory board for BMS; International Response Committee for Eloquent-1 trial (completed); and stock or stock options for AbbVie, Abbott, Biogen, BMS, Gilead, Ionis, Johnson & Johnson, and Lilly Sorrento. C.H.L. reports payment for chairing meeting for BMS and advisory board participation for Janssen, Antengene, and BMS. L.A.H. reports study funding to institution from Seattle Genetics, Sanofi, Millennium-Takeda, BMS, Merck, Janssen, and iTeos Therapeutics; payment from UpToDate; data safety monitoring board or advisory board participation for iTeas Therapeutics (no payment); and leadership or fiduciary role in other board, society, committee, or advocacy group participation (no payment) for NCCN Guidelines MM, Waldenström macroglobulinemia, and Primary Systemic Amyloidosis. M.S. reports speakers bureau participation for BMS and GSK. T.N. reports research support (unrelated to the current study) to the institution from Novartis (clinical trial) and Karyopharm (drug supply only). L.D.A. Jr reports research funding from GSK, Janssen, BMS, Celgene, Amgen, AbbVie, and Regeneron; consulting fees from GSK, Janssen, BMS, Celgene, Karyopharm, Beigene, Amgen, and AbbVie; and data safety monitoring board or advisory board participation for Prothena. M.Q. reports payment from Amgen Inc, Bioline Rx Ld., Janssen Scientific, Angiocrine Bioscience, and NexImmune Inc. N.S. reports as voluntary clinical faculty at the University of California, San Francisco, in the Hematology-Bone Marrow Transplant Clinic. S.K.K. reports research funding for clinical trials to the institution from AbbVie, Amgen, BMS, Carsgen, Janssen, AstraZeneca, Novartis, Roche-Genentech, Takeda, Tenebio, and Molecular Templates; consulting/advisory board participation (with no personal payments) from AbbVie, Amgen, BMS, Janssen, Roche-Genentech, Takeda, AstraZeneca, Bluebird Bio, Epizyme, Secura Biotherapeutics, Monterosa Therapeutics, and Trillium; and consulting/advisory board participation (with personal payment) from Oncopeptides, Beigene, Antengene, and GLH Pharma. S.Z.U. reports grants or contracts from Amgen, Array BioPharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx, and Takeda; and consulting fees from AbbVie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio; and payment or honoraria from Amgen, Beigene, BMS, Janssen, and Sanofi.
Figures
Comment in
-
Autologous transplant and second malignancies in MM.Blood Adv. 2023 Jun 27;7(12):2731-2732. doi: 10.1182/bloodadvances.2023010051. Blood Adv. 2023. PMID: 37318936 Free PMC article. No abstract available.
References
-
- Child JA, Morgan GJ, Davies FE, et al. Medical Research Council Adult Leukaemia Working Party High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. - PubMed
-
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–97. - PubMed
-
- Paul B, Lipe B, Ocio EM, Usmani SZ. Induction therapy for newly diagnosed multiple myeloma. Am Soc Clin Oncol Educ Book. 2019;39:e176–e186. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

