Difficulties in ensuring review quality performed by committees under the Act on the Safety of Regenerative Medicine in Japan
- PMID: 36827977
- PMCID: PMC10031296
- DOI: 10.1016/j.stemcr.2023.01.013
Difficulties in ensuring review quality performed by committees under the Act on the Safety of Regenerative Medicine in Japan
Erratum in
-
Difficulties in ensuring review quality performed by committees under the Act on the Safety of Regenerative Medicine in Japan.Stem Cell Reports. 2023 May 9;18(5):1247. doi: 10.1016/j.stemcr.2023.04.006. Stem Cell Reports. 2023. PMID: 37163983 Free PMC article. No abstract available.
Abstract
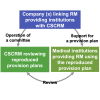
We outlined five studies regarding the quality of the review by committees based on the Act on the Safety of Regenerative Medicine. The findings raise serious concerns about the independence, integrity, and quality of reviews of therapeutic plans by these committees with inappropriately close relationships to medical institutions and companies.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interests We conducted the studies commissioned by the MHLW. This paper is based on a part of the report published as outcomes of the commissioned projects.
Figures
References
-
- Health Science Council . Report “Building a framework for safety assurance and promotion of RM”. 2013. Expert committee on safety assurance and promotion of RM.https://www.mhlw.go.jp/stf/shingi/2r9852000002zggt-att/2r9852000002zgkl.pdf
-
- Health Science Council, Regenerative Medicine Evaluation Committee. The interim report on the review of the law for enforcement of act on the safety of regenerative medicine 5 years after its enforcement (2019). https://www.mhlw.go.jp/content/000581069.pdf.