Monocytes re-enter the bone marrow during fasting and alter the host response to infection
- PMID: 36827982
- PMCID: PMC10101885
- DOI: 10.1016/j.immuni.2023.01.024
Monocytes re-enter the bone marrow during fasting and alter the host response to infection
Abstract
Diet profoundly influences physiology. Whereas over-nutrition elevates risk for disease via its influence on immunity and metabolism, caloric restriction and fasting appear to be salutogenic. Despite multiple correlations observed between diet and health, the underlying biology remains unclear. Here, we identified a fasting-induced switch in leukocyte migration that prolongs monocyte lifespan and alters susceptibility to disease in mice. We show that fasting during the active phase induced the rapid return of monocytes from the blood to the bone marrow. Monocyte re-entry was orchestrated by hypothalamic-pituitary-adrenal (HPA) axis-dependent release of corticosterone, which augmented the CXCR4 chemokine receptor. Although the marrow is a safe haven for monocytes during nutrient scarcity, re-feeding prompted mobilization culminating in monocytosis of chronologically older and transcriptionally distinct monocytes. These shifts altered response to infection. Our study shows that diet-in particular, a diet's temporal dynamic balance-modulates monocyte lifespan with consequences for adaptation to external stressors.
Keywords: bone marrow; corticosterone; fasting; hematopoiesis; hypothalamus; infection; monocyte.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.N. has received funding or material research support from Alnylam, Biotronik, CSL Behring, GlycoMimetics, GSK, Medtronic, Novartis, and Pfizer as well as consulting fees from Lilly, Biogen, Gimv, IFM Therapeutics, Molecular Imaging, Sigilon, and Verseau Therapeutics. F.K.S. has received funds or material research support from Novartis, Partner Therapeutics, Pfizer, and Verseau Therapeutics.
Figures

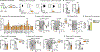

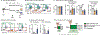


Comment in
-
Fasting affects monocyte lifespan and migration.Nat Rev Immunol. 2023 Apr;23(4):203. doi: 10.1038/s41577-023-00863-z. Nat Rev Immunol. 2023. PMID: 36918664 No abstract available.
-
Old and "hangry" monocytes turn from friend to foe under assault.Immunity. 2023 Apr 11;56(4):747-749. doi: 10.1016/j.immuni.2023.03.013. Immunity. 2023. PMID: 37044065
-
Energy saver: Monocytes hibernate in bone marrow upon fasting.Cell Metab. 2023 May 2;35(5):734-736. doi: 10.1016/j.cmet.2023.04.008. Cell Metab. 2023. PMID: 37137288
-
The immune hunger games: the effects of fasting on monocytes.Cell Mol Immunol. 2023 Oct;20(10):1098-1100. doi: 10.1038/s41423-023-01033-w. Epub 2023 May 10. Cell Mol Immunol. 2023. PMID: 37165013 Free PMC article. No abstract available.
References
-
- Dehghan M et al. (2017). Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 390, 2050–2062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

