Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists
- PMID: 36828370
- PMCID: PMC9958666
- DOI: 10.3390/tomography9010018
Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists
Abstract
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) comprise a heterogeneous group of neoplasms, which derive from cells of the diffuse neuroendocrine system that specializes in producing hormones and neuropeptides and arise in most cases sporadically and, to a lesser extent, in the context of complex genetic syndromes. Furthermore, they are primarily nonfunctioning, while, in the case of insulinomas, gastrinomas, glucagonomas, vipomas, and somatostatinomas, they produce hormones responsible for clinical syndromes. The GEP-NEN tumor grade and cell differentiation may result in different clinical behaviors and prognoses, with grade one (G1) and grade two (G2) neuroendocrine tumors showing a more favorable outcome than grade three (G3) NET and neuroendocrine carcinoma. Two critical issues should be considered in the NEN diagnostic workup: first, the need to identify the presence of the tumor, and, second, to define the primary site and evaluate regional and distant metastases. Indeed, the primary site, stage, grade, and function are prognostic factors that the radiologist should evaluate to guide prognosis and management. The correct diagnostic management of the patient includes a combination of morphological and functional evaluations. Concerning morphological evaluations, according to the consensus guidelines of the European Neuroendocrine Tumor Society (ENETS), computed tomography (CT) with a contrast medium is recommended. Contrast-enhanced magnetic resonance imaging (MRI), including diffusion-weighted imaging (DWI), is usually indicated for use to evaluate the liver, pancreas, brain, and bones. Ultrasonography (US) is often helpful in the initial diagnosis of liver metastases, and contrast-enhanced ultrasound (CEUS) can solve problems in characterizing the liver, as this tool can guide the biopsy of liver lesions. In addition, intraoperative ultrasound is an effective tool during surgical procedures. Positron emission tomography (PET-CT) with FDG for nonfunctioning lesions and somatostatin analogs for functional lesions are very useful for identifying and evaluating metabolic receptors. The detection of heterogeneity in somatostatin receptor (SSTR) expression is also crucial for treatment decision making. In this narrative review, we have described the role of morphological and functional imaging tools in the assessment of GEP-NENs according to current major guidelines.
Keywords: PET; computed tomography; diagnosis; gastroenteropancreatic; magnetic resonance; neoplasms; neuroendocrine; radiology; somatostatin receptor imaging; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
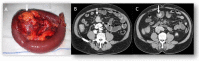
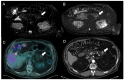
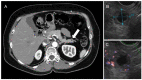
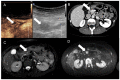
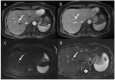
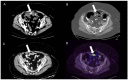
Similar articles
-
ESR Essentials: role of PET/CT in neuroendocrine tumors-practice recommendations by the European Society for Hybrid, Molecular and Translational Imaging.Eur Radiol. 2025 Apr;35(4):1903-1912. doi: 10.1007/s00330-024-11095-7. Epub 2024 Oct 10. Eur Radiol. 2025. PMID: 39387873 Free PMC article. Review.
-
Correlation and Comparison of Somatostatin Receptor Type 2 Immunohistochemical Scoring Systems with 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography Imaging in Gastroenteropancreatic Neuroendocrine Neoplasms.Neuroendocrinology. 2022;112(4):358-369. doi: 10.1159/000517530. Epub 2021 Jun 2. Neuroendocrinology. 2022. PMID: 34077939
-
[Neuroendocrine tumors of the pancreas].Radiologie (Heidelb). 2023 Dec;63(12):894-899. doi: 10.1007/s00117-023-01231-7. Epub 2023 Nov 10. Radiologie (Heidelb). 2023. PMID: 37947864 Review. German.
-
Use and perceived utility of [18 F]FDG PET/CT in neuroendocrine neoplasms: A consensus report from the European Neuroendocrine Tumor Society (ENETS) Advisory Board Meeting 2022.J Neuroendocrinol. 2024 Jan;36(1):e13359. doi: 10.1111/jne.13359. Epub 2023 Dec 14. J Neuroendocrinol. 2024. PMID: 38097193
-
Gastroenteropancreatic neuroendocrine tumours (GEP-NET) - Imaging and staging.Best Pract Res Clin Endocrinol Metab. 2016 Jan;30(1):45-57. doi: 10.1016/j.beem.2016.01.003. Epub 2016 Jan 20. Best Pract Res Clin Endocrinol Metab. 2016. PMID: 26971843 Review.
Cited by
-
Multimodal Imaging Approach to MEN-1 Syndrome-Associated Tumors.Diagnostics (Basel). 2025 May 3;15(9):1164. doi: 10.3390/diagnostics15091164. Diagnostics (Basel). 2025. PMID: 40361982 Free PMC article. Review.
-
Role of B-Mode and Contrast-Enhanced Ultrasound in the Diagnostic Workflow of Gastro-Entero-Pancreatic Neuroendocrine Tumors (GEP-NETs).Cancers (Basel). 2025 Jun 4;17(11):1879. doi: 10.3390/cancers17111879. Cancers (Basel). 2025. PMID: 40507359 Free PMC article. Review.
-
[Imaging of pancreatic neuroendocrine tumors].Radiologie (Heidelb). 2024 Jul;64(7):559-567. doi: 10.1007/s00117-024-01316-x. Epub 2024 May 24. Radiologie (Heidelb). 2024. PMID: 38789854 German.
-
Innovative imaging approaches for neuroendocrine tumor characterization: Combined dual energy CT and perfusion protocol implementation.Radiol Case Rep. 2024 Jul 20;19(10):4225-4231. doi: 10.1016/j.radcr.2024.06.063. eCollection 2024 Oct. Radiol Case Rep. 2024. PMID: 39101023 Free PMC article.
-
Defining MRI Superiority over CT for Colorectal and Neuroendocrine Liver Metastases.Cancers (Basel). 2023 Oct 23;15(20):5109. doi: 10.3390/cancers15205109. Cancers (Basel). 2023. PMID: 37894475 Free PMC article.
References
-
- Yao J., Hassan M., Phan A., Dagohoy C., Leary C., Mares J., Abdalla E., Fleming J., Vauthey J.-N., Rashid A., et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. - DOI - PubMed
-
- Chiti G., Grazzini G., Flammia F., Matteuzzi B., Tortoli P., Bettarini S., Pasqualini E., Granata V., Busoni S., Messserini L., et al. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A radiomic model to predict tumor grade. Radiol. Med. 2022;127:1234. doi: 10.1007/s11547-022-01529-x. - DOI - PubMed
-
- Benedetti G., Mori M., Panzeri M., Barbera M., Palumbo D., Sini C., Muffatti F., Andreasi V., Steidler S., Doglioni C., et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Med. 2021;126:745–760. doi: 10.1007/s11547-021-01333-z. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

