Active Nuclear Import of Mammalian Cell-Expressible DNA Origami
- PMID: 36828394
- PMCID: PMC9999407
- DOI: 10.1021/jacs.2c12733
Active Nuclear Import of Mammalian Cell-Expressible DNA Origami
Abstract
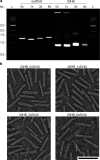
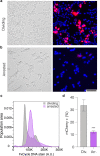
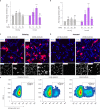
DNA origami enables the creation of complex 3D shapes from genetic material. Future uses could include the delivery of genetic instructions to cells, but nuclear import remains a major barrier to gene delivery due to the impermeability of the nuclear membrane. Here we realize active nuclear import of DNA origami objects in dividing and chemically arrested mammalian cells. We developed a custom DNA origami single-strand scaffold featuring a mammalian-cell expressible reporter gene (mCherry) and multiple Simian virus 40 (SV40) derived DNA nuclear targeting sequences (DTS). Inclusion of the DTS within DNA origami rescued gene expression in arrested cells, indicating that active transport into the nucleus occurs. Our work successfully adapts mechanisms known from viruses to promote the cellular expression of genetic instructions encoded within DNA origami objects.
Conflict of interest statement
The authors declare the following competing financial interest(s): A patent has been filed by TUM.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

