Determination of Microcystins in Fish Tissue by ELISA and MALDI-TOF MS Using a Highly Specific Single Domain Antibody
- PMID: 36828400
- PMCID: PMC9966346
- DOI: 10.3390/toxins15020084
Determination of Microcystins in Fish Tissue by ELISA and MALDI-TOF MS Using a Highly Specific Single Domain Antibody
Abstract
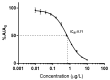
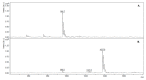
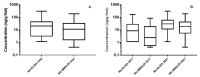
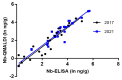
The development of simple, reliable, and cost-effective methods is critically important to study the spatial and temporal variation of microcystins (MCs) in the food chain. Nanobodies (Nbs), antigen binding fragments from camelid antibodies, present valuable features for analytical applications. Their small antigen binding site offers a focused recognition of small analytes, reducing spurious cross-reactivity and matrix effects. A high affinity and broad cross-reactivity anti-MCs-Nb, from a llama antibody library, was validated in enzyme linked immunosorbent assay (ELISA), and bound to magnetic particles with an internal standard for pre-concentration in quantitative-matrix-assisted laser desorption ionization-time of flight mass spectrometry (Nb-QMALDI MS). Both methods are easy and fast; ELISA provides a global result, while Nb-QMALDI MS allows for the quantification of individual congeners and showed excellent performance in the fish muscle extracts. The ELISA assay range was 1.8-29 ng/g and for Nb-QMALDI, it was 0.29-29 ng/g fish ww. Fifty-five fish from a MC-containing dam were analyzed by both methods. The correlation ELISA/sum of the MC congeners by Nb-QMALDI-MS was very high (r Spearman = 0.9645, p < 0.0001). Using ROC curves, ELISA cut-off limits were defined to accurately predict the sum of MCs by Nb-QMALDI-MS (100% sensitivity; ≥89% specificity). Both methods were shown to be simple and efficient for screening MCs in fish muscle to prioritize samples for confirmatory methods.
Keywords: ELISA; MALDI-TOF; fish; microcystins; nanobody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Oriented Functionalization of Magnetic Beads with in Vivo Biotinylated Nanobodies for Rapid MALDI-TOF MS Ultrasensitive Quantitation of Microcystins in Biological Samples.Anal Chem. 2019 Aug 6;91(15):9925-9931. doi: 10.1021/acs.analchem.9b01596. Epub 2019 Jul 24. Anal Chem. 2019. PMID: 31291093
-
Development and validation of a rapid method for microcystins in fish and comparing LC-MS/MS results with ELISA.Anal Bioanal Chem. 2011 Nov;401(8):2617-30. doi: 10.1007/s00216-011-5345-0. Epub 2011 Sep 1. Anal Bioanal Chem. 2011. PMID: 21881880
-
An integrated strategy for rapid and accurate determination of free and cell-bound microcystins and related peptides in natural blooms by liquid chromatography-electrospray-high resolution mass spectrometry and matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry using both positive and negative ionization modes.J Chromatogr A. 2015 Aug 14;1407:76-89. doi: 10.1016/j.chroma.2015.06.022. Epub 2015 Jun 19. J Chromatogr A. 2015. PMID: 26141269
-
Recent developments in the methods of quantitative analysis of microcystins.J Biochem Mol Toxicol. 2020 Dec;34(12):e22582. doi: 10.1002/jbt.22582. Epub 2020 Jul 14. J Biochem Mol Toxicol. 2020. PMID: 32662914 Review.
-
Analysis of protein glycation products by matrix-assisted laser desorption ionization time-of-flight mass spectrometry.Curr Med Chem. 2004 Aug;11(16):2185-93. doi: 10.2174/0929867043364649. Curr Med Chem. 2004. PMID: 15279557 Review.
Cited by
-
A review on camelid nanobodies with potential application in veterinary medicine.Vet Res Commun. 2024 Aug;48(4):2051-2068. doi: 10.1007/s11259-024-10432-x. Epub 2024 Jun 13. Vet Res Commun. 2024. PMID: 38869749 Review.
References
-
- Bormans M., Savar V., Legrand B., Mineaud E., Robert E., Lance E., Amzil Z. Cyanobacteria and Cyanotoxins in Estuarine Water and Sediment. Aquat. Ecol. 2020;54:625–640. doi: 10.1007/s10452-020-09764-y. - DOI
-
- Paerl H.W., Havens K.E., Xu H., Zhu G., McCarthy M.J., Newell S.E., Scott J.T., Hall N.S., Otten T.G., Qin B. Mitigating Eutrophication and Toxic Cyanobacterial Blooms in Large Lakes: The Evolution of a Dual Nutrient (N and P) Reduction Paradigm. Hydrobiologia. 2020;847:4359–4375. doi: 10.1007/s10750-019-04087-y. - DOI
-
- Metcalf J.S., Banack S.A., Wessel R.A., Lester M., Pim J.G., Cassani J.R., Cox P.A. Toxin Analysis of Freshwater Cyanobacterial and Marine Harmful Algal Blooms on the West Coast of Florida and Implications for Estuarine Environments. Neurotox. Res. 2021;39:27–35. doi: 10.1007/s12640-020-00248-3. - DOI - PMC - PubMed
-
- Preece E.P., Hardy F.J., Moore B.C., Bryan M. A Review of Microcystin Detections in Estuarine and Marine Waters: Environmental Implications and Human Health Risk. Harmful Algae. 2017;61:31–45. doi: 10.1016/j.hal.2016.11.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

