A Practical Approach in Refining Binary Outcome for Treatment Effect of COVID-19 According to Geographical Diversity
- PMID: 36828499
- PMCID: PMC9964378
- DOI: 10.3390/tropicalmed8020083
A Practical Approach in Refining Binary Outcome for Treatment Effect of COVID-19 According to Geographical Diversity
Abstract
The recent COVID-19 pandemic has drawn attention to health and economics worldwide. Initially, diseases only ravage local populations, while a pandemic could aggravate global economic burdens. Lopinavir/Ritonavir is an anti-HIV drug that was used on small scale patients during SARS, but its effectiveness for COVID-19 treatment is still unclear. Previous studies or meta-analysis have retrieved clinical data of subgroup analysis to evaluate the efficacy and safety of Lopinavir/Ritonavir for the treatment of COVID-19 in a few affected regions. However, geographical diversity and small number of studies bias correction were not achieved in such subgroup analysis of published meta-analysis. The present study demonstrates a practical approach in refining the binary outcome for COVID-19 treatment of Lopinavir/Ritonavir according to geographical location diversity and small number of studies (less than or equal to five) for subgroup analysis. After performing practical approach, the risk of adverse event with LPV/RTV for treatment of COVID-19 becomes nonsignificant compared to previous meta-analysis. Furthermore, we also notice heterogeneity of random effect of meta-analysis may be declined after proposed adjustment. In conclusion, proposed practical approach is recommend for performing a subgroup analysis to avoid concentration in a single geographical location and small number of studies bias.
Keywords: COVID-19; Lopinavir/Ritonavir; efficacy; geographical diversity; meta-analysis; safety; small number of studies bias.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
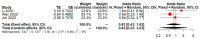


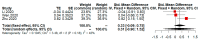
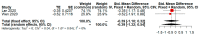
References
-
- Loyola S., Cano-Pérez E., Torres-Pacheco J., Malambo-Garcia D., Gomez R., Gomez-Camargo D. Epidemiology of COVID-19 in Individuals under 18 Years Old in Cartagena, Colombia: An Ecological Study of the First 14 Months of the Pandemic. Trop. Med. Infect. Dis. 2022;7:107. doi: 10.3390/tropicalmed7060107. - DOI - PMC - PubMed
-
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

