Colchicine and high-intensity rosuvastatin in the treatment of non-critically ill patients hospitalised with COVID-19: a randomised clinical trial
- PMID: 36828654
- PMCID: PMC9971831
- DOI: 10.1136/bmjopen-2022-067910
Colchicine and high-intensity rosuvastatin in the treatment of non-critically ill patients hospitalised with COVID-19: a randomised clinical trial
Abstract
Objective: To evaluate the effect of colchicine and high-intensity rosuvastatin in addition to standard of care on the progression of COVID-19 disease in hospitalised patients.
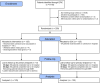
Design: A pragmatic, open-label, multicentre, randomised controlled trial conducted from October 2020 to September 2021. Follow-up was conducted at 30 and 60 days. The electronic medical record was used at all stages of the trial including screening, enrolment, randomisation, event ascertainment and follow-up.
Setting: Four centres in the Yale New Haven Health System.
Participants: Non-critically ill hospitalised patients with COVID-19.
Interventions: Patients were randomised 1:1 to either colchicine plus high-intensity rosuvastatin in addition to standard of care versus standard of care alone. Assigned treatment was continued for the duration of index hospitalisation or 30 days, whichever was shorter.
Primary and secondary outcome measures: The prespecified primary endpoint was progression to severe COVID-19 disease (new high-flow or non-invasive ventilation, mechanical ventilation, need for vasopressors, renal replacement therapy or extracorporeal membrane oxygenation, or death) or arterial/venous thromboembolic events (ischaemic stroke, myocardial infarction, deep venous thrombosis or pulmonary embolism) evaluated at 30 days.
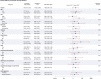
Results: Among the 250 patients randomised in this trial (125 to each arm), the median age was 61 years, 44% were women, 15% were Black and 26% were Hispanic/Latino. As part of the standard of care, patients received remdesivir (87%), dexamethasone (92%), tocilizumab (18%), baricitinib (2%), prophylactic/therapeutic anticoagulation (98%) and aspirin (91%). The trial was terminated early by the data and safety monitoring board for futility. No patients were lost to follow-up due to electronic medical record follow-up. There was no significant difference in the primary endpoint at 30 days between the active arm and standard of care arm (15.2% vs 8.8%, respectively, p=0.17).
Conclusions: In this small, open-label, randomised trial of non-critically ill hospitalised patients with COVID-19, the combination of colchicine and rosuvastatin in addition to standard of care did not appear to reduce the risk of progression of COVID-19 disease or thromboembolic events, although the trial was underpowered due to a lower-than-expected event rate. The trial leveraged the power of electronic medical records for efficiency and improved follow-up and demonstrates the utility of incorporating electronic medical records into future trials.
Trial registration: NCT04472611.
Keywords: COVID-19; clinical pharmacology; internal medicine; respiratory infections.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Feb 5;22(1):116. doi: 10.1186/s13063-021-05072-4. Trials. 2021. PMID: 33546739 Free PMC article.
-
Subcutaneous Sarilumab in hospitalised patients with moderate-severe COVID-19 infection compared to the standard of care (SARCOVID): a structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Sep 9;21(1):772. doi: 10.1186/s13063-020-04588-5. Trials. 2020. PMID: 32907638 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Real-world causal evidence for planned predictive enrichment in critical care trials: A scoping review.Acta Anaesthesiol Scand. 2024 Jan;68(1):16-25. doi: 10.1111/aas.14321. Epub 2023 Aug 30. Acta Anaesthesiol Scand. 2024. PMID: 37649412
-
Evidence review for colchicine: NICE COVID-19 rapid guideline: managing COVID-19: Evidence review F.London: National Institute for Health and Care Excellence (NICE); 2021 Nov. London: National Institute for Health and Care Excellence (NICE); 2021 Nov. PMID: 39383271 Free Books & Documents. Review. No abstract available.
Cited by
-
Adapting power calculations to include a superiority margin: what are the implications?Biochem Med (Zagreb). 2024 Feb 15;34(1):010101. doi: 10.11613/BM.2024.010101. Biochem Med (Zagreb). 2024. PMID: 38361735 Free PMC article. Review.
-
Drug-Drug Interactions and the Clinical Tolerability of Colchicine Among Patients With COVID-19: A Secondary Analysis of the COLCORONA Randomized Clinical Trial.JAMA Netw Open. 2024 Sep 3;7(9):e2431309. doi: 10.1001/jamanetworkopen.2024.31309. JAMA Netw Open. 2024. PMID: 39240567 Free PMC article. Clinical Trial.
-
Atorvastatin for reduction of 28-day mortality in severe and critical COVID-19 patients: a randomized controlled trial.Respir Res. 2024 Feb 22;25(1):97. doi: 10.1186/s12931-024-02732-2. Respir Res. 2024. PMID: 38389078 Free PMC article. Clinical Trial.
-
Oxygen Saturation in Hospitalized COVID-19 Patients and Its Relation to Colchicine Treatment: A Retrospective Cohort Study with an Updated Systematic Review.Medicina (Kaunas). 2023 May 12;59(5):934. doi: 10.3390/medicina59050934. Medicina (Kaunas). 2023. PMID: 37241167 Free PMC article.
References
-
- Marconi VC, Ramanan AV, de Bono S, et al. . Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021;9:1407–18. 10.1016/S2213-2600(21)00331-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
