Metastatic colorectal cancer: mechanisms and emerging therapeutics
- PMID: 36828759
- PMCID: PMC10365888
- DOI: 10.1016/j.tips.2023.01.003
Metastatic colorectal cancer: mechanisms and emerging therapeutics
Abstract
Metastatic colorectal cancer (mCRC) remains a lethal disease with an approximately 14% 5-year survival rate. While early-stage colorectal cancer (CRC) can be cured by surgery with or without adjuvant chemotherapy, mCRC cannot be eradicated due to a large burden of disseminated cancer cells comprising therapy-resistant metastasis-competent cells. To address this gap, recent studies have focused on further elucidating the molecular mechanisms underlying colorectal metastasis and recognizing the limitations of available therapeutic interventions. In this review, we discuss newfound factors that regulate CRC cell dissemination and colonization of distant organs, such as genetic mutations, identification of metastasis-initiating cells (MICs), epithelial-mesenchymal transition (EMT), and the tumor microenvironment (TME). We also review current treatments for mCRC, therapeutic regimens undergoing clinical trials, and trending preclinical studies being investigated to target treatment-resistant mCRC.
Keywords: cancer therapeutics; colorectal cancer; immunotherapy; metastasis; targeted therapy.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no interests.
Figures

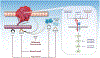


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

