Citrullination modulates antigen processing and presentation by revealing cryptic epitopes in rheumatoid arthritis
- PMID: 36828807
- PMCID: PMC9958131
- DOI: 10.1038/s41467-023-36620-y
Citrullination modulates antigen processing and presentation by revealing cryptic epitopes in rheumatoid arthritis
Abstract
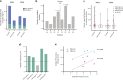
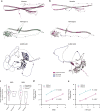
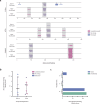
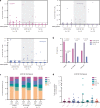
Cryptic peptides, hidden from the immune system under physiologic conditions, are revealed by changes to MHC class II processing and hypothesized to drive the loss of immune tolerance to self-antigens in autoimmunity. Rheumatoid arthritis (RA) is an autoimmune disease characterized by immune responses to citrullinated self-antigens, in which arginine residues are converted to citrullines. Here, we investigate the hypothesis that citrullination exposes cryptic peptides by modifying protein structure and proteolytic cleavage. We show that citrullination alters processing and presentation of autoantigens, resulting in the generation of a unique citrullination-dependent repertoire composed primarily of native sequences. This repertoire stimulates T cells from RA patients with anti-citrullinated protein antibodies more robustly than controls. The generation of this unique repertoire is achieved through altered protease cleavage and protein destabilization, rather than direct presentation of citrulline-containing epitopes, suggesting a novel paradigm for the role of protein citrullination in the breach of immune tolerance in RA.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: E.D. is an inventor on a licensed patent (US patent no. 8,975,033) and licensed provisional patent (US patent no. 62/481,158) related to the use of antibodies to PAD3 and PAD2, respectively, in identifying clinically informative disease subsets in RA, and has received consulting fees from Celgene and Bristol Myers Squibb and research support from Pfizer, Celgene, and Bristol Myers Squibb outside of this work. The remaining authors declare no competing interests.
Figures


Comment in
-
How does citrullination contribute to RA autoantibody development?Nat Rev Rheumatol. 2023 Jun;19(6):329-330. doi: 10.1038/s41584-023-00959-9. Nat Rev Rheumatol. 2023. PMID: 37016165 No abstract available.
Similar articles
-
Immune recognition of citrullinated epitopes.Immunology. 2016 Oct;149(2):131-8. doi: 10.1111/imm.12640. Epub 2016 Aug 17. Immunology. 2016. PMID: 27531825 Free PMC article. Review.
-
A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis.J Exp Med. 2013 Nov 18;210(12):2569-82. doi: 10.1084/jem.20131241. Epub 2013 Nov 4. J Exp Med. 2013. PMID: 24190431 Free PMC article.
-
Citrullination only infrequently impacts peptide binding to HLA class II MHC.PLoS One. 2017 May 8;12(5):e0177140. doi: 10.1371/journal.pone.0177140. eCollection 2017. PLoS One. 2017. PMID: 28481943 Free PMC article.
-
Extensive Citrullination Promotes Immunogenicity of HSP90 through Protein Unfolding and Exposure of Cryptic Epitopes.J Immunol. 2016 Sep 1;197(5):1926-36. doi: 10.4049/jimmunol.1600162. Epub 2016 Jul 22. J Immunol. 2016. PMID: 27448590 Free PMC article.
-
Microbial pathways to subvert host immunity generate citrullinated neoantigens targeted in rheumatoid arthritis.Curr Opin Struct Biol. 2022 Aug;75:102423. doi: 10.1016/j.sbi.2022.102423. Epub 2022 Jul 11. Curr Opin Struct Biol. 2022. PMID: 35834948 Free PMC article. Review.
Cited by
-
Citrullination of matrisomal proteins in health and diseases.Philos Trans R Soc Lond B Biol Sci. 2023 Nov 20;378(1890):20220244. doi: 10.1098/rstb.2022.0244. Epub 2023 Oct 2. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37778384 Free PMC article. Review.
-
Emerging biochemical, microbial and immunological evidence in the search for why HLA-B∗27 confers risk for spondyloarthritis.Cell Chem Biol. 2025 Jan 16;32(1):12-24. doi: 10.1016/j.chembiol.2024.07.012. Epub 2024 Aug 20. Cell Chem Biol. 2025. PMID: 39168118 Review.
-
Unravelling the Oral-Gut Axis: Interconnection Between Periodontitis and Inflammatory Bowel Disease, Current Challenges, and Future Perspective.J Crohns Colitis. 2024 Aug 14;18(8):1319-1341. doi: 10.1093/ecco-jcc/jjae028. J Crohns Colitis. 2024. PMID: 38417137 Free PMC article. Review.
-
How does citrullination contribute to RA autoantibody development?Nat Rev Rheumatol. 2023 Jun;19(6):329-330. doi: 10.1038/s41584-023-00959-9. Nat Rev Rheumatol. 2023. PMID: 37016165 No abstract available.
-
Exploring the Role of the Microbiome in Rheumatoid Arthritis-A Critical Review.Microorganisms. 2024 Jul 9;12(7):1387. doi: 10.3390/microorganisms12071387. Microorganisms. 2024. PMID: 39065155 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

