An analgesic pathway from parvocellular oxytocin neurons to the periaqueductal gray in rats
- PMID: 36828816
- PMCID: PMC9958129
- DOI: 10.1038/s41467-023-36641-7
An analgesic pathway from parvocellular oxytocin neurons to the periaqueductal gray in rats
Abstract
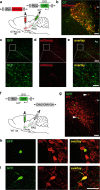
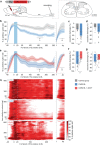
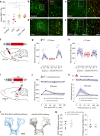
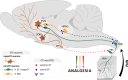
The hypothalamic neuropeptide oxytocin (OT) exerts prominent analgesic effects via central and peripheral action. However, the precise analgesic pathways recruited by OT are largely elusive. Here we discovered a subset of OT neurons whose projections preferentially terminate on OT receptor (OTR)-expressing neurons in the ventrolateral periaqueductal gray (vlPAG). Using a newly generated line of transgenic rats (OTR-IRES-Cre), we determined that most of the vlPAG OTR expressing cells targeted by OT projections are GABAergic. Ex vivo stimulation of parvocellular OT axons in the vlPAG induced local OT release, as measured with OT sensor GRAB. In vivo, optogenetically-evoked axonal OT release in the vlPAG of as well as chemogenetic activation of OTR vlPAG neurons resulted in a long-lasting increase of vlPAG neuronal activity. This lead to an indirect suppression of sensory neuron activity in the spinal cord and strong analgesia in both female and male rats. Altogether, we describe an OT-vlPAG-spinal cord circuit that is critical for analgesia in both inflammatory and neuropathic pain models.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Althammer, F. & Grinevich, V. Diversity of oxytocin neurons: beyond magno- and parvocellular cell types? J. Neuroendocrinol.30, e12549 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

