Assessment of the clinical and analytical performance of the Aptima SARS-CoV-2 assay using the VALCOR protocol
- PMID: 36829164
- PMCID: PMC9951132
- DOI: 10.1186/s12985-023-01986-4
Assessment of the clinical and analytical performance of the Aptima SARS-CoV-2 assay using the VALCOR protocol
Abstract
Background: The COVID-19 pandemic highlighted the importance of diagnostic testing against curbing the spread of SARS-CoV-2. The urgent need and scale for diagnostic tools resulted in manufacturers of SARS-CoV-2 assays receiving emergency authorization that lacked robust analytical or clinical evaluation. As it is highly likely that testing for SARS-CoV-2 will continue to play a central role in public health, the performance characteristics of assays should be evaluated to ensure reliable diagnostic outcomes are achieved.
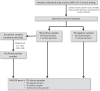
Methods: VALCOR or "VALidation of SARS-CORona Virus-2 assays" is a study protocol designed to set up a framework for test validation of SARS-CoV-2 virus assays. Using clinical samples collated from VALCOR, the performance of Aptima SARS-CoV-2 assay was assessed against a standard comparator assay. Diagnostic test parameters such as sensitivity, specificity and overall per cent agreement were calculated for the clinical performance of Aptima SARS-CoV-2 assay.
Results: A total of 180 clinical samples were tested with an addition of 40 diluted clinical specimens to determine the limit of detection. When compared to the standard comparator assay Aptima had a sensitivity of 100.0% [95% CI 95.9-100.0] and specificity of 96.7% [95% CI 90.8-99.3]. The overall percent agreement was 98.3% with an excellent Cohen's coefficient of κ = 0.967 [95% CI 0.929-1.000]. For the limit of detection, Aptima was able to detect all of the diluted clinical samples.
Conclusion: In conclusion. validation of Aptima SARS-CoV-2 assay using clinical samples collated through the VALCOR protocol showed excellent test performance. Additionally, Aptima demonstrated high analytical sensitivity by detecting all diluted clinical samples corresponding to a low limit of detection.
Keywords: Aptima; COVID-19; Diagnostic test accuracy; Quality control; RT-PCR; SARS-CoV-2; Test validation; Transcription mediated amplification; VALCOR.
© 2023. The Author(s).
Conflict of interest statement
VALCOR is a researcher induced study protocol where manufacturers can participate under the condition of providing test kits and covering research costs. Researchers do not receive any financial advantage by collaborating in VALCOR. Any mention of commercial products does not imply recommendation or endorsement by the authors.
Figures
Similar articles
-
Assessment of the clinical and analytical performance of three Seegene Allplex SARS-CoV-2 assays within the VALCOR framework.Microbiol Spectr. 2024 Feb 6;12(2):e0239723. doi: 10.1128/spectrum.02397-23. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189291 Free PMC article.
-
VALCOR: a protocol for the validation of SARS-corona virus-2 assays.Arch Public Health. 2022 Mar 29;80(1):98. doi: 10.1186/s13690-022-00869-4. Arch Public Health. 2022. PMID: 35351191 Free PMC article.
-
Analytical and Clinical Comparison of Three Nucleic Acid Amplification Tests for SARS-CoV-2 Detection.J Clin Microbiol. 2020 Aug 24;58(9):e01134-20. doi: 10.1128/JCM.01134-20. Print 2020 Aug 24. J Clin Microbiol. 2020. PMID: 32571894 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
Cited by
-
Evaluation of SARS-CoV-2 Detection Systems Using Clinical Samples and Standard Material: A Comparative Study.Diagnostics (Basel). 2023 Jun 13;13(12):2046. doi: 10.3390/diagnostics13122046. Diagnostics (Basel). 2023. PMID: 37370941 Free PMC article.
-
Ensure the accuracy and consistency of biochemical analyzer test results: Chemometrics for instrument and inter-instrument item comparison in Chinese hospital laboratory.Heliyon. 2024 Jan 6;10(1):e24306. doi: 10.1016/j.heliyon.2024.e24306. eCollection 2024 Jan 15. Heliyon. 2024. PMID: 38268603 Free PMC article.
-
Effects of Frailty Syndrome on Osteoporosis, Focusing on the Mediating Effect of Muscle Strength and Balance in Community-Dwelling Older Adults (≥60 years) in Iran: Results From the Amirkola Health and Aging Project Cohort Study.Geriatr Orthop Surg Rehabil. 2024 Jul 25;15:21514593241264647. doi: 10.1177/21514593241264647. eCollection 2024. Geriatr Orthop Surg Rehabil. 2024. PMID: 39070931 Free PMC article.
-
Assessment of the clinical and analytical performance of three Seegene Allplex SARS-CoV-2 assays within the VALCOR framework.Microbiol Spectr. 2024 Feb 6;12(2):e0239723. doi: 10.1128/spectrum.02397-23. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189291 Free PMC article.
-
Biomarkers in diagnosing and therapeutic monitoring of tuberculosis: a review.Ann Med. 2024 Dec;56(1):2386030. doi: 10.1080/07853890.2024.2386030. Epub 2024 Aug 3. Ann Med. 2024. PMID: 39097795 Free PMC article. Review.
References
-
- World Health Organization (WHO). Weekly operational update on COVID-19. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... Accessed 03 Aug 2022.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous