Efficacy and safety of TNF inhibitors in the treatment of juvenile idiopathic arthritis: a systematic literature review
- PMID: 36829225
- PMCID: PMC9951426
- DOI: 10.1186/s12969-023-00798-8
Efficacy and safety of TNF inhibitors in the treatment of juvenile idiopathic arthritis: a systematic literature review
Abstract
Objective: A systematic literature review was conducted to summarize efficacy and safety data from studies that evaluated tumor necrosis factor inhibitors in patients with juvenile idiopathic arthritis (JIA).
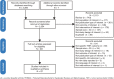
Methods: Relevant publications were identified via online searches (cutoff: March 16, 2021). After screening search results, outcome data were extracted if the treatment arm included ≥ 30 patients. Outcomes were described narratively, with efficacy assessed by JIA-American College of Rheumatology (ACR) response criteria and safety assessed by the incidence of serious adverse events (SAEs) per 100 patient-years (100PY).
Results: Among 87 relevant publications included in the qualitative synthesis, 19 publications described 13 clinical trials. Across the 13 trials, the percentages of patients who achieved JIA-ACR30/50/70/90 responses at Week 12 with adalimumab ranged 71-94%, 68-90%, 55-61%, and 39-42%, respectively; with etanercept (Week 12), 73-94%, 53-78%, 36-59%, and 28%; with golimumab (Week 16), 89%, 79%, 66%, and 36%; and with infliximab (Week 14), 64%, 50%, and 22% (JIA-ACR90 not reported). SAE incidence across all time points ranged 0-13.7 SAE/100PY for adalimumab, 0-20.0 SAE/100PY for etanercept, and 10.4-24.3 SAE/100PY for golimumab (1 study). SAE incidence could not be estimated from the 2 infliximab publications.
Conclusion: Tumor necrosis factor inhibitors are effective and well tolerated in the treatment of JIA, but additional evidence from head-to-head studies and over longer periods of time, especially in the context of the transition from pediatric to adult care, would be useful.
© 2023. The Author(s).
Conflict of interest statement
GH declares serving on advisory boards and receiving research funds and honoraria from AbbVie, Chugai, GSK, MSD, Pfizer, Roche, and Sobi. KM declares serving on advisory boards and receiving honoraria from AbbVie, GSK, Novartis, Pfizer, Roche, and Sanofi. CR was an employee of Envision Pharma Group at the time the study was conducted and was a paid consultant to Pfizer in connection with the development of the manuscript. ACHD declares former employment with Pfizer, the sponsor of this systematic literature review, and holds stock and/or stock options with Pfizer. CB is an employee of Pfizer and holds stock and/or stock options with Pfizer. NR declares receiving honoraria for consultancies and serving on speakers bureaux for Ablynx, AstraZeneca-Medimmune, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, EMD Serono, GSK, Hoffmann-La Roche, Janssen, MSD, Novartis, Pfizer, R-Pharma, Sanofi, Servier, Sinergie, and Sobi; and is a full-time public employee of the IRCCS Istituto Giannina Gaslini that has received contributions from Bristol Myers Squibb, Eli Lilly, GSK, Hoffmann-La Roche, Janssen, Novartis, Pfizer, and Sobi, all of which have been invested in the research activities of the hospital in a fully independent manner and without any commitment to third parties.
References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical