Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract
- PMID: 36829475
- PMCID: PMC9953177
- DOI: 10.3390/biology12020196
Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract
Abstract
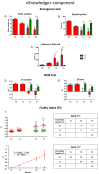
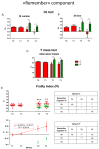
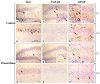
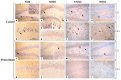
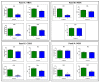
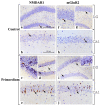
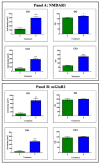
Brain aging is a crucial risk factor for several neurodegenerative disorders and dementia. The most affected cognitive function is memory, worsening early during aging. Inflammation and oxidative stress are known to have a role in pathogenesis of cognitive impairments, and a link exists between aging/frailty and immunosenescence/inflammaging. Based on anti-aging properties, medicinal mushrooms represent a source to develop medicines and functional foods. In particular, Hericium erinaceus (He) displays several actions ranging from boosting the immune system to fighting senescence, due to its active ingredients/metabolites. Among these, Ergothioneine (ERGO) is known as the longevity vitamin. Currently, we demonstrated the efficacy of an ERGO-rich He primordium extract (He2) in preventing cognitive decline in a murine model of aging. We focused on recognition memory deterioration during aging, monitored through spontaneous behavioral tests assessing both memory components and frailty index. A parallel significant decrease in key markers of inflammation and oxidative stress, i.e., IL6, TGFβ1, GFAP, Nrf2, SOD1, COX2, NOS2, was revealed in the hippocampus by immunohistochemistry, accompanied by an enhancement of NMDAR1and mGluR2, crucially involved in glutamatergic neurotransmission. In summary, we disclosed a selective, preventive and neuroprotective effect of He2 on aged hippocampus, both on recognition memory as well on inflammation/oxidative stress/glutamate receptors expression.
Keywords: Hericium erinaceus primordium; aging; ergothioneine; frailty; hippocampus; inflammation; medicinal mushroom supplementation; memory; neuroprotection; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Searching for a Longevity Food, We Bump into Hericium erinaceus Primordium Rich in Ergothioneine: The "Longevity Vitamin" Improves Locomotor Performances during Aging.Nutrients. 2022 Mar 11;14(6):1177. doi: 10.3390/nu14061177. Nutrients. 2022. PMID: 35334834 Free PMC article.
-
Neuroprotective Metabolites of Hericium erinaceus Promote Neuro-Healthy Aging.Int J Mol Sci. 2021 Jun 15;22(12):6379. doi: 10.3390/ijms22126379. Int J Mol Sci. 2021. PMID: 34203691 Free PMC article.
-
Hericium erinaceus Improves Recognition Memory and Induces Hippocampal and Cerebellar Neurogenesis in Frail Mice during Aging.Nutrients. 2019 Mar 27;11(4):715. doi: 10.3390/nu11040715. Nutrients. 2019. PMID: 30934760 Free PMC article.
-
The Monkey Head Mushroom and Memory Enhancement in Alzheimer's Disease.Cells. 2022 Jul 24;11(15):2284. doi: 10.3390/cells11152284. Cells. 2022. PMID: 35892581 Free PMC article. Review.
-
Antitumor, Anti-Inflammatory and Antiallergic Effects of Agaricus blazei Mushroom Extract and the Related Medicinal Basidiomycetes Mushrooms, Hericium erinaceus and Grifola frondosa: A Review of Preclinical and Clinical Studies.Nutrients. 2020 May 8;12(5):1339. doi: 10.3390/nu12051339. Nutrients. 2020. PMID: 32397163 Free PMC article. Review.
Cited by
-
Neurotrophic and Neuroprotective Effects of Hericium erinaceus.Int J Mol Sci. 2023 Nov 3;24(21):15960. doi: 10.3390/ijms242115960. Int J Mol Sci. 2023. PMID: 37958943 Free PMC article. Review.
-
Theoretical Potential of Hericium Erinaceus Supplementation as an Add-On to Antipsychotics in Chronic and Treatment-Resistant Schizophrenia.Psychopharmacol Bull. 2025 Feb 3;55(2):41-59. Psychopharmacol Bull. 2025. PMID: 39935672 Review.
-
Osteogenesis Imperfecta: A Look into the Cerebellum of the Brtl Murine Model.Mol Neurobiol. 2025 Jul 22. doi: 10.1007/s12035-025-05205-9. Online ahead of print. Mol Neurobiol. 2025. PMID: 40694281
-
Ergothioneine-Mediated Neuroprotection of Human iPSC-Derived Dopaminergic Neurons.Antioxidants (Basel). 2024 Jun 5;13(6):693. doi: 10.3390/antiox13060693. Antioxidants (Basel). 2024. PMID: 38929132 Free PMC article.
-
Acute Effects of Naturally Occurring Guayusa Tea and Nordic Lion's Mane Extracts on Cognitive Performance.Nutrients. 2023 Dec 6;15(24):5018. doi: 10.3390/nu15245018. Nutrients. 2023. PMID: 38140277 Free PMC article. Clinical Trial.
References
-
- Magnusson K., Sehlin D., Syvänen S., Svedberg M.M., Philipson O., Söderberg L., Tegerstedt K., Holmquist M., Gellerfors P., Tolmachev V., et al. Specific Uptake of an Amyloid-β Protofibril-Binding Antibody-Tracer in AβPP Transgenic Mouse Brain. J. Alzheimers Dis. 2013;37:29–40. doi: 10.3233/JAD-130029. - DOI - PubMed
-
- Gasiorowska A., Wydrych M., Drapich P., Zadrozny M., Steczkowska M., Niewiadomski W., Niewiadomska G. The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front. Aging Neurosci. 2021;13:654931. doi: 10.3389/fnagi.2021.654931. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

